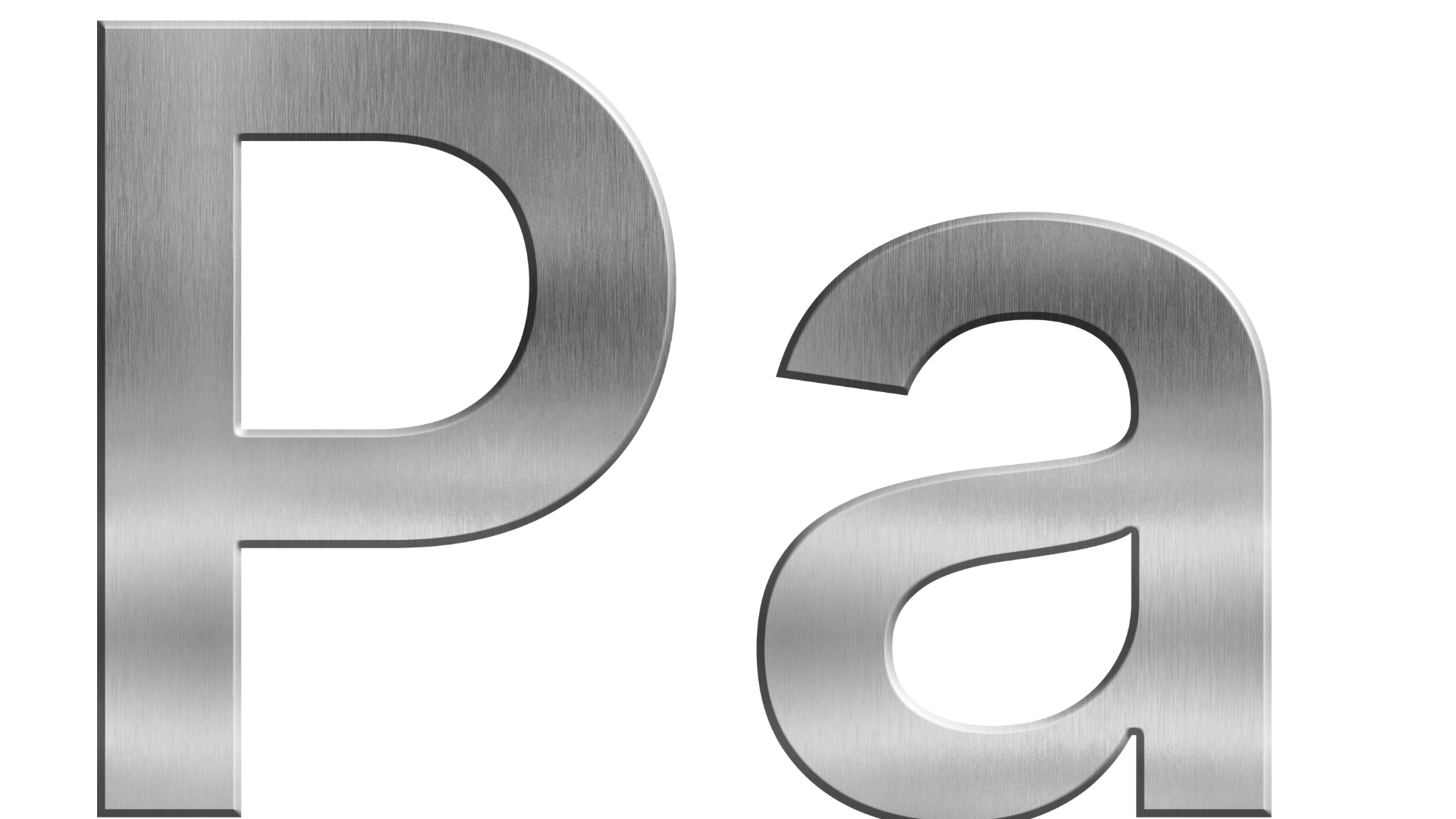
In the realm of science, engineering, and everyday measurement, precision is critical. Whether it’s the force exerted by air in a car tire or the pressure of blood flowing through arteries, accurate pressure measurement is essential. One of the most universally accepted ways to measure pressure is through the pascal in SI units — the internationally recognized metric unit for pressure.
The SI unit system, short for Système International d’Unités, provides a standardized framework for measurement across disciplines and countries. Among the various derived units within this system, the pascal plays a vital role in defining pressure and stress. Named after the 17th-century French mathematician and physicist Blaise Pascal, this unit forms the cornerstone of pressure measurement in scientific and industrial applications worldwide.
But what exactly is a pascal in SI units, and why is it so important?
To put it simply, one pascal is defined as one newton per square meter (1 Pa = 1 N/m²). This definition reflects the concept of pressure as a force distributed over an area. Whether engineers are calculating the load-bearing capabilities of a bridge, meteorologists are forecasting atmospheric changes, or doctors are interpreting blood pressure readings, the pascal in SI units provides a consistent and reliable unit of measure. Its application spans disciplines, industries, and geographic boundaries.
Understanding the pascal in SI units is more than memorizing a conversion factor. It’s about grasping how pressure behaves in different systems, how it’s quantified, and why standardization is essential in both everyday life and highly technical environments. While alternative pressure units like psi (pounds per square inch), bar, or torr are still in use in some regions or legacy systems, the SI unit of pascal ensures compatibility and clarity in global communication.
Over time, the pascal in SI units has become a foundational concept in physics and engineering textbooks, research publications, ISO standards, and even mobile weather apps. Its widespread adoption is a testament to the power of SI units in unifying the scientific community under a common language of measurement.
In this article, we will explore the pascal in SI units in detail — from its historical origins and scientific definition to real-world applications, conversion examples, visualizations, and how it fits into the broader SI framework. Whether you’re a student, engineer, technician, or just curious about how pressure is measured, this comprehensive guide will provide clarity and practical insights into the world of pascals.
Let’s begin by understanding the foundations of the SI unit system before diving into the specifics of the pascal in SI units.
What Are SI Units?
To understand the pascal in SI units, it’s essential first to grasp the broader context of what SI units are and why they matter. The International System of Units (SI) is the modern form of the metric system and the most widely used system of measurement in the world. It provides a coherent, standardized framework for scientists, engineers, manufacturers, and everyday users across the globe.

A Brief History of SI Units
The roots of the SI system date back to the late 18th century in France, during the Age of Enlightenment. As scientific knowledge expanded rapidly, so did the need for a uniform, rational system of measurement. The metric system was introduced in 1795, and later, in 1960, the Système International d’Unités was established by the 11th General Conference on Weights and Measures (CGPM).
Today, the SI system is maintained and continually refined by the International Bureau of Weights and Measures (BIPM), ensuring consistency and accuracy in global measurements.
The Seven Base SI Units
The SI system is built on seven fundamental base units, from which all other units — including derived units like the pascal in SI units — are constructed:
| Quantity | SI Base Unit | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Thermodynamic temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
Each of these base units represents a precisely defined physical quantity and serves as the foundation for more complex measurements.
Derived Units and the Pascal
Derived units are combinations of base units that represent additional quantities, such as velocity, force, and pressure. The pascal in SI units is one such derived unit. It is defined using the newton (N) — itself a derived unit — and square meter (m²):
1 Pa = 1 N/m²
1 N = 1 kg·m/s², so:
1 Pa = 1 kg/(m·s²)
This definition shows that a pascal in SI units quantifies the amount of force (in newtons) applied per unit area (in square meters). It bridges multiple base units — mass, length, and time — into a practical and measurable concept of pressure.
Why Use SI Units?
The global adoption of SI units offers several advantages:
-
Uniformity: All countries and industries can use the same measurement standards.
-
Precision: SI definitions are based on fundamental constants, ensuring reproducibility and consistency.
-
Ease of Conversion: The decimal-based nature of SI units makes them easy to convert between multiples (e.g., pascal to kilopascal).
-
Interdisciplinary Communication: From physics and chemistry to medicine and engineering, the SI system allows for clear communication across scientific domains.
Using the pascal in SI units ensures that pressure measurements are not only accurate but also universally understandable — whether you’re working in a European lab, an American aerospace facility, or a weather station in Asia.
Defining the Pascal in SI Units
The pascal in SI units is the internationally recognized unit of pressure, named in honor of the French physicist and mathematician Blaise Pascal. In the SI system, pressure is a derived quantity that expresses how force is distributed over an area. The formal definition of the pascal reflects this relationship:
1 Pascal (Pa) = 1 Newton per square meter (N/m²)
This simple equation — Pa = N/m² — defines the pascal as one newton of force applied uniformly over one square meter of area. The equation highlights how the pascal in SI units is rooted in base and derived units of the SI system.
Breaking Down the Units Behind the Pascal
To understand what a pascal in SI units really means, we must analyze the components of the definition:
-
Newton (N): The SI unit of force, defined as the force required to accelerate a mass of 1 kilogram by 1 meter per second squared (1 N = 1 kg·m/s²).
-
Square Meter (m²): The SI unit of area.
Putting it all together:
1 Pa = 1 N/m² = 1 (kg·m/s²) / m² = 1 kg/(m·s²)
Thus, one pascal in SI units is equal to one kilogram per meter per second squared. This shows that the pascal combines mass, length, and time — three of the SI base quantities — into one practical unit for measuring pressure or mechanical stress.
What Is Pressure?
Pressure is a measure of how much force is acting on a specific area. It’s an essential concept in physics, chemistry, biology, meteorology, and engineering. For example:
-
A tire inflated to 250,000 pascals (or 250 kilopascals) is exerting 250,000 newtons of force per square meter of its inner surface.
-
Atmospheric pressure at sea level is approximately 101,325 pascals, also known as 1 atmosphere (atm).
The pascal in SI units enables scientists and engineers to quantify this pressure consistently and accurately.
Understanding Scale: Pascal, Kilopascal, and Beyond
Because one pascal is a relatively small unit of pressure, larger multiples are often used in practice:
| Unit | Symbol | Value in Pascals |
|---|---|---|
| Pascal | Pa | 1 Pa |
| Kilopascal | kPa | 1,000 Pa |
| Megapascal | MPa | 1,000,000 Pa |
| Gigapascal | GPa | 1,000,000,000 Pa |
Each of these larger units is derived by multiplying the pascal in SI units by powers of ten, which keeps calculations and reporting concise and practical — especially in high-pressure applications like hydraulics or materials testing.
Comparison with Other Pressure Units
Although the pascal in SI units is globally accepted, several other units are still in use, especially in older or imperial-based systems. Here’s how they compare:
| Unit | Equivalent in Pascals |
|---|---|
| 1 atm | 101,325 Pa |
| 1 bar | 100,000 Pa |
| 1 psi (pound/in²) | 6,894.76 Pa |
| 1 torr | 133.322 Pa |
| 1 mmHg | 133.322 Pa |
These conversions illustrate why understanding the pascal in SI units is critical — it provides the universal reference point from which all other pressure measurements can be converted or calibrated.
Why Is the Pascal the SI Unit of Pressure?
The decision to define pressure in terms of newtons per square meter was guided by simplicity and logical consistency. The SI system strives for interconnectivity between units, allowing for seamless mathematical and conceptual alignment across disciplines. Because pressure is fundamentally a force applied over an area, using the newton (force) and the square meter (area) naturally leads to the pascal in SI units.
By standardizing on the pascal, the SI system enables engineers, scientists, and technicians worldwide to communicate without ambiguity — a crucial requirement in fields where accuracy can impact safety, cost, and performance.
Historical Background of the Pascal
To truly appreciate the significance of the pascal in SI units, it’s helpful to explore its historical roots — both in terms of scientific discovery and international standardization. The story of the pascal is deeply intertwined with the life and contributions of Blaise Pascal, and the evolution of modern measurement systems.
Blaise Pascal: The Namesake of the Unit
Born in 1623, Blaise Pascal was a French mathematician, physicist, and philosopher who made groundbreaking contributions to fluid mechanics, hydrodynamics, and pressure theory. Among his most influential experiments was his demonstration of the existence of atmospheric pressure and how it changes with altitude.
In one famous experiment, Pascal’s brother-in-law carried a barometer up the Puy de Dôme mountain in France. As the elevation increased, the mercury column dropped, confirming Pascal’s theory that atmospheric pressure decreases with height. This experiment helped establish pressure as a measurable and variable force — not just a theoretical concept.
To honor his work, the SI unit of pressure was named “pascal” during the 14th General Conference on Weights and Measures (CGPM) in 1971. This designation not only celebrated Pascal’s contributions but also helped bring clarity and consistency to the field of pressure measurement.
Before the Pascal: A Landscape of Confusion
Before the adoption of the pascal in SI units, pressure was measured in a wide array of units — depending on industry, geography, and application. These included:
-
Millimeters of mercury (mmHg) in medicine and meteorology
-
Pounds per square inch (psi) in the U.S. and other imperial-based systems
-
Atmospheres (atm) in chemistry and physics
-
Bars and torrs in engineering and vacuum science
Each of these units served a purpose but created confusion in global communication and required complex conversions. The need for a universal pressure unit became increasingly important as international trade, research, and engineering projects expanded across borders.
Adoption into the SI System
The formal inclusion of the pascal in SI units was a landmark moment. It helped consolidate pressure measurement into a form compatible with the rest of the SI system. Because the pascal is derived directly from the newton and the square meter — both well-established SI units — it fit seamlessly into the structure.
The move toward SI units, including the pascal, was driven by organizations like:
-
BIPM (Bureau International des Poids et Mesures)
-
ISO (International Organization for Standardization)
-
IEC (International Electrotechnical Commission)
Together, these bodies ensured that the pascal in SI units would be standardized, traceable, and globally recognized in engineering specifications, scientific publications, and technical documentation.
Why the Historical Context Matters
Understanding the historical evolution of the pascal in SI units helps explain why it is so widely trusted today. It represents more than just a quantity — it reflects centuries of scientific progress and a global commitment to measurement consistency. Today, when engineers use pascals to test pressure vessels, or when meteorologists report atmospheric pressure in hectopascals, they are using a unit built upon the legacy of Blaise Pascal and the rigor of the international SI framework.
Practical Applications of the Pascal in SI Units
The pascal in SI units is far more than a theoretical construct—it is a practical, widely-used unit of pressure that plays a vital role in industries, laboratories, and even everyday life. Thanks to its universality and consistency, the pascal allows professionals from different fields and regions to communicate pressure-related data without confusion or the need for complex conversions.
Let’s explore the many areas where the pascal in SI units is applied, and how it contributes to safety, performance, and innovation.
1. Engineering and Structural Design
In civil, mechanical, and aerospace engineering, pressure and stress analysis are foundational. The pascal in SI units is used to express internal and external pressures acting on structures such as:
-
Bridges and buildings (e.g., wind loads, water pressure)
-
Pressure vessels (e.g., boilers, tanks)
-
Aircraft fuselages and wings (e.g., cabin pressure and aerodynamic loads)
Engineers often use kilopascals (kPa) or megapascals (MPa) to describe these forces, since a single pascal is too small for large-scale systems.
2. Meteorology and Atmospheric Science
Weather forecasting relies heavily on pressure measurements. Atmospheric pressure is commonly reported in hectopascals (hPa), where:
1 hPa = 100 pascals
For example, a typical sea-level atmospheric pressure of 1013.25 hPa equals 101,325 pascals. The pascal in SI units provides meteorologists with a standardized and accurate way to model weather patterns and predict storms.
3. Medicine and Physiology
In medical diagnostics, precise pressure measurement is essential. Here are some examples:
-
Blood pressure: Although often reported in mmHg, it can be converted to pascals for scientific reporting.
-
Ventilators and respiratory systems: Use cmH₂O or Pa to regulate and monitor airway pressure.
-
Intracranial pressure: Measured in mmHg or converted to pascals for SI-compliant reports.
Medical equipment manufacturers commonly use the pascal in SI units to meet regulatory standards and ensure patient safety across global markets.
4. Fluid Dynamics and Process Control
In industrial systems that involve gases and liquids under pressure, such as:
-
Pipelines
-
Hydraulic presses
-
Chemical reactors
…the pascal in SI units is used to calculate:
-
Fluid pressure drop
-
Pump specifications
-
Valve pressure ratings
For instance, a hydraulic system may operate at 20 MPa, or 20,000,000 pascals, showing how the pascal in SI units accommodates extreme pressures.
5. Automotive and Transportation Systems
Modern vehicles use pressure sensors and monitoring systems calibrated in pascals or kilopascals to control:
-
Tire pressure monitoring systems (TPMS)
-
Engine intake manifold pressure
-
Brake line hydraulic pressure
Automotive technicians frequently refer to the pascal in SI units when interpreting sensor data from diagnostics tools.
6. Environmental Monitoring and Safety
Pressure measurement is crucial for environmental and workplace safety:
-
Leak detection in gas pipelines
-
Air quality monitoring in cleanrooms
-
Explosion-proof pressure equipment testing
Agencies and manufacturers rely on pressure gauges, transducers, and alarms calibrated using the pascal in SI units to ensure compliance with ISO and safety regulations.
7. Research and Development
From laboratories studying material behavior under pressure to semiconductor fabs requiring exact vacuum pressure levels, pascal in SI units is used in:
-
Scientific research papers and journals
-
Sensor calibration and benchmarking
-
Precision-controlled vacuum chambers
It’s not uncommon to find research data reported in micropascals (μPa) or gigapascals (GPa) depending on the scale.
8. Everyday Consumer Applications
Even outside professional settings, the pascal in SI units finds its way into daily use:
-
Weather apps showing air pressure in hPa (hectopascals)
-
Air compressors labeled in kPa or MPa
-
Bicycle tire gauges marked in psi and kPa
Consumers may not be aware they’re using the pascal in SI units, but the standardized labeling ensures consistent product performance and safety.
Why Standardization Matters
Using the pascal in SI units across industries eliminates ambiguity, supports international trade, and ensures that engineers in Japan, scientists in Germany, and manufacturers in the U.S. are speaking the same technical language.
This standardization reduces errors, improves efficiency, and strengthens interoperability in everything from oil rigs to smartphones.
Conversions Involving Pascal in SI Units
While the pascal in SI units is the standard for measuring pressure globally, professionals often need to convert it into other commonly used pressure units — especially when working with legacy systems, industry-specific standards, or regional conventions. Understanding these conversions is crucial for ensuring precision and avoiding costly errors in fields like engineering, medicine, and aviation.
1. Common Unit Conversions for Pascal
Below is a quick-reference chart showing how the pascal in SI units compares to other popular units of pressure:
| Unit | Symbol | Equivalent in Pascals (Pa) |
|---|---|---|
| Bar | bar | 100,000 Pa |
| Atmosphere | atm | 101,325 Pa |
| Millimeter of Mercury | mmHg | 133.322 Pa |
| Torr | torr | 133.322 Pa |
| Pound per square inch | psi | 6,894.76 Pa |
| Kilopascal | kPa | 1,000 Pa |
| Megapascal | MPa | 1,000,000 Pa |
| Hectopascal | hPa | 100 Pa |
These conversions show how the pascal in SI units acts as the base reference, from which all other pressure units can be systematically derived.
2. Conversion Formulas
To convert between the pascal in SI units and other units, use the following formulas:
-
Pascal to Bar:
Pressure (bar) = Pressure (Pa) ÷ 100,000 -
Pascal to Atmosphere (atm):
Pressure (atm) = Pressure (Pa) ÷ 101,325 -
Pascal to psi:
Pressure (psi) = Pressure (Pa) ÷ 6,894.76 -
Pascal to mmHg or Torr:
Pressure (mmHg) = Pressure (Pa) ÷ 133.322
To convert back to pascals, multiply by the same factor instead of dividing.
3. Real-World Conversion Examples
Example 1:
Convert 500,000 Pa to bar:
→ 500,000 ÷ 100,000 = 5 bar
Example 2:
Convert 250,000 Pa to psi:
→ 250,000 ÷ 6,894.76 ≈ 36.26 psi
Example 3:
Convert 1 atm to pascals:
→ 1 × 101,325 = 101,325 Pa
These examples help illustrate how the pascal in SI units bridges various systems, making it easy to translate pressure measurements into familiar formats.
4. Engineering Applications of Unit Conversion
In technical fields, converting the pascal in SI units is often required when:
-
Designing hydraulic or pneumatic systems where psi is still used
-
Reading European and U.S. product manuals interchangeably
-
Working with software simulations that require inputs in kPa or MPa
-
Validating test reports that use different pressure unit conventions
Engineers, technicians, and students alike benefit from fluency in converting from pascal in SI units to alternate units to ensure compatibility and accuracy in documentation.
5. Digital Tools and Resources
Today, many professionals rely on tools to handle conversions:
-
Online unit converters
-
Engineering calculator apps
-
Spreadsheet functions (e.g., Excel CONVERT function)
Despite the convenience of these tools, understanding the manual math behind converting the pascal in SI units fosters a stronger grasp of pressure relationships and increases confidence in interpreting data.
6. Tips for Accurate Conversion
-
Always double-check the unit labels — “kPa” is not the same as “Pa.”
-
Be cautious with rounding — small differences can become large errors in high-pressure systems.
-
When working with vacuum pressures or gauge pressures, be clear about reference points (absolute vs. relative pressure).
-
Always convert to SI base units (Pa) before applying formulas in physics or engineering problems.
Understanding these conversions not only enhances accuracy in work involving pressure — it reinforces the versatility and universality of the pascal in SI units in global applications.
Visualizing Pascal in SI Units
For many learners and professionals, understanding a unit mathematically is only part of the equation. To truly internalize what pressure means, it’s helpful to visualize the pascal in SI units in physical terms. After all, saying “one newton per square meter” is accurate — but what does that feel like? How much pressure is a pascal in practice?
1. How Much Is One Pascal?
Let’s start small. One pascal in SI units is defined as one newton of force applied over one square meter of area. A newton is about the weight of a small apple. So:
1 Pa = the pressure exerted by a small apple spread over an area of 1 m².
That’s a tiny amount of pressure — practically imperceptible. This is why in most real-world applications, we often use larger multiples like kilopascals (kPa) or megapascals (MPa).
2. Common Real-World Pressure Examples (in Pascals)
To make the pascal in SI units more tangible, here are some everyday pressures and their equivalent values:
| Example | Approximate Pressure |
|---|---|
| Atmospheric pressure at sea level | 101,325 Pa (101.3 kPa) |
| Car tire pressure | 200,000–300,000 Pa (200–300 kPa) |
| Blood pressure (systolic) | ~16,000 Pa (120 mmHg) |
| Pressure at 10 meters underwater | ~100,000 Pa (1 bar) |
| Hydraulic press (industrial) | 10–100 MPa |
| Jet engine turbine pressure | Up to 500 MPa |
| Vacuum in a semiconductor fab | <1 Pa |
These examples show the broad scale of the pascal in SI units — from ultra-low vacuum levels to extreme high-pressure environments in advanced machinery.
3. Visual Diagrams for Better Understanding
Though we can’t show physical diagrams here, these are excellent visual tools you can include in your article:
-
Diagram 1: A weight (1 newton) resting on a 1 m² surface to illustrate 1 Pa.
-
Diagram 2: A vertical scale showing pressure ranges from 1 Pa to 1 GPa, with icons (e.g., tire, diver, jet engine) marking typical values.
-
Diagram 3: Pressure distribution around a balloon, with internal pressure in kPa.
Visualizing the pascal in SI units this way helps bridge the gap between abstract theory and real-world phenomena.
4. Comparing Pascal to Other Units Visually
Another way to make the pascal in SI units relatable is to compare it to more commonly known units:
-
1 psi = 6,894.76 Pa → Much higher than a single pascal
-
1 mmHg = 133.322 Pa → Used in blood pressure
-
1 bar = 100,000 Pa → About the same as pressure at 10 meters underwater
These relationships can be plotted on a horizontal bar chart or logarithmic scale to show how pressure units align, emphasizing why the pascal in SI units is essential as a common baseline.
5. The Human Feel of Pressure
For a more intuitive sense:
-
Touching a desk with a finger applies several thousand pascals of pressure due to the small contact area.
-
Standing on one foot applies tens of thousands of pascals due to body weight and contact area.
-
Pressing a thumbtack into a surface can reach hundreds of thousands of pascals at the point of contact.
These tactile experiences help us relate to what the pascal in SI units means on a physical level.
Conclusion of Visualization
By visualizing the pascal in SI units, we can appreciate its range, flexibility, and importance. Whether describing the pressure in our bodies or in aerospace systems, the pascal offers a standard measurement language that scales from nearly imperceptible to extreme.
Pascal in SI Units in Scientific Research and Industry Standards
The pascal in SI units is not just a tool for classroom exercises or theoretical models—it is a cornerstone of international standards used in scientific research, manufacturing processes, industrial safety, and product testing. Because pressure affects the performance, safety, and behavior of systems in countless applications, having a standardized and precise unit like the pascal is indispensable across disciplines and industries.
1. Scientific Research and Laboratory Use
In scientific laboratories—whether studying physics, chemistry, biology, or materials science—precise pressure control is often essential. Experiments involving:
-
Gas laws and thermodynamics
-
Vacuum chambers and low-pressure systems
-
High-pressure reactions in chemistry
-
Biological cell environments
… all require accurate pressure measurements and reporting. The pascal in SI units is the universal language for recording and sharing this data in peer-reviewed journals, international databases, and laboratory records.
For example:
-
A materials researcher testing the compressive strength of alloys may report results in megapascals (MPa).
-
A chemist measuring vapor pressure may report results in kilopascals (kPa) or pascals.
Using the pascal in SI units ensures repeatability and comparability across different institutions and countries.
2. Industrial Standards and Compliance
Global industries—including oil & gas, aerospace, automotive, pharmaceuticals, and food processing—operate under stringent standards that rely on pressure measurements expressed in SI units. Regulatory bodies and standard organizations such as:
-
ISO (International Organization for Standardization)
-
ASME (American Society of Mechanical Engineers)
-
IEC (International Electrotechnical Commission)
-
NIST (National Institute of Standards and Technology)
…mandate the use of SI-based measurements, making the pascal in SI units essential for compliance.
Common examples include:
-
ISO 4126: Safety valves, pressure relief devices — specified in pascals
-
ASME BPVC (Boiler & Pressure Vessel Code): All pressure testing conducted in pascals, kPa, or MPa
-
Pharmaceutical cleanroom pressure differentials: Controlled and logged using pascals or hPa
3. Instrumentation and Sensor Calibration
Pressure sensors, gauges, transducers, and transmitters are critical in almost every industrial process. Devices are calibrated in pascals or their multiples to ensure traceability to international standards.
Calibration labs regularly use reference pressure standards expressed in pascals to ensure devices comply with metrological standards.
For instance:
-
A pressure transducer may operate within 0–500 kPa and must be calibrated to within ±1 kPa.
-
In semiconductor cleanrooms, differential pressure sensors must detect changes as low as ±10 Pa to maintain air quality control.
These systems depend on the consistency of the pascal in SI units to maintain safety, efficiency, and product integrity.
4. Safety Regulations and Testing
Industrial environments are often subject to extreme pressure conditions, from vacuum systems to high-pressure vessels. Safety codes require:
-
Hydrostatic testing of tanks and pipes (MPa range)
-
Leak testing of gas pipelines (kPa or Pa)
-
Burst testing of components under sudden overpressure
These evaluations are logged, certified, and archived using pascal in SI units to meet local and international legal standards.
5. Academic and Educational Standards
Universities and technical institutions teach fluid mechanics, thermodynamics, and physical sciences using the pascal in SI units to ensure students are trained in globally accepted practices. Textbooks, exams, and simulations all default to SI units to build a strong foundation for future professionals.
Even educational software and calculators now use pascals as the default pressure unit in physics simulations and lab software.
6. Cross-Disciplinary Communication
In multidisciplinary teams—combining engineers, physicists, chemists, and data scientists—the pascal in SI units eliminates ambiguity and translation errors. Whether working on:
-
Environmental testing of electronics
-
Fuel cell development
-
Climate modeling
-
High-pressure sterilization systems
…standardization through the pascal in SI units ensures accurate and consistent communication.
Conclusion of This Section
The use of the pascal in SI units in scientific and industrial standards is foundational to modern engineering and research. It ensures that from pressure gauges in aerospace systems to lab reports in academic journals, everyone speaks the same technical language. This consistency is key to progress, safety, and global collaboration.
Challenges and Misconceptions
While the pascal in SI units is a clear and standardized way to measure pressure, its practical usage is not always straightforward. In fact, many learners, professionals, and even seasoned engineers encounter common challenges and misconceptions when dealing with this unit. Understanding these can help ensure more accurate communication, safer designs, and better educational outcomes.
1. Mistaking Pascal for a Base Unit
One common misconception is believing that the pascal in SI units is a base unit in the SI system. In reality, the pascal is a derived unit, defined from more fundamental quantities:
1 Pa = 1 N/m²
1 N = 1 kg·m/s², thus:
1 Pa = 1 kg/(m·s²)
Failing to understand this derivation can lead to confusion when performing unit analysis in equations involving force, area, or pressure.
2. Misinterpreting the Scale of a Pascal
Another challenge is appreciating the actual magnitude of a single pascal. Because 1 Pa is such a small unit of pressure, it is rarely used on its own in practical contexts. Beginners may mistakenly believe that a few pascals represent substantial pressure, when in fact:
-
1 Pa is roughly the pressure exerted by a dollar bill resting on a table.
-
Most engineering applications deal with kilopascals (kPa) or megapascals (MPa) instead.
This misunderstanding often results in poor design choices or misreadings of pressure data.
3. Confusion Between Gauge Pressure and Absolute Pressure
The pascal in SI units can be used to express either gauge pressure or absolute pressure — but they’re not the same:
-
Gauge pressure: Measured relative to atmospheric pressure.
-
Absolute pressure: Measured relative to a perfect vacuum.
For example:
-
A tire pressure of 250 kPa gauge is actually ~351 kPa absolute, assuming atmospheric pressure is ~101 kPa.
Mistaking one for the other can lead to critical errors in system design or instrumentation.
4. Converting Incorrectly Between Units
While conversion formulas are simple in theory, mistakes often happen due to:
-
Using incorrect factors (e.g., confusing psi with bar)
-
Neglecting unit prefixes (e.g., mixing up Pa, kPa, and MPa)
-
Failing to account for significant digits
For example:
Converting 2.5 MPa to kPa should yield 2,500 kPa, but users might mistakenly report it as 250 kPa by dropping a zero.
Having a clear grasp of metric prefixes is essential when working with the pascal in SI units.
5. Regional and Legacy Unit Overlap
In many countries, non-SI pressure units like psi, mmHg, or atm are still widely used. This overlap can cause confusion, especially when:
-
Reading manuals or standards from different regions.
-
Switching between metric and imperial tools.
-
Comparing historical data with SI-compliant documentation.
Professionals must be vigilant in converting to and from the pascal in SI units to ensure accuracy and safety.
6. Misuse in Software or Spreadsheets
Spreadsheets, simulation tools, or sensors may default to pressure units without explicitly labeling them. If a value appears as “1000,” is that Pa, kPa, or psi?
Misinterpreting this context—especially when importing/exporting data—can severely skew results. Always verify the unit base in tools that handle pressure input/output.
7. Overlooking Temperature Effects on Pressure
Many learners fail to connect pressure to temperature, especially in gas systems governed by the Ideal Gas Law (PV = nRT). While the pascal in SI units measures pressure independently, real-world systems often require temperature correction for accurate readings, especially in:
-
Pneumatic systems
-
HVAC applications
-
Laboratory experiments
Understanding these thermodynamic relationships is critical for full and correct use of pascal in SI units.
Conclusion of Challenges Section
Despite its simplicity and standardization, the pascal in SI units is frequently misunderstood or misused due to scaling issues, legacy unit systems, or unclear documentation. By being aware of these challenges, engineers, students, and technicians can more confidently use pascals in calculations, conversions, and real-world applications.
Educational Tools and Resources
Understanding the pascal in SI units is a critical skill for students, engineers, technicians, and educators working with pressure-related systems. Fortunately, a wide range of tools and resources are available to make learning and applying this unit easier, faster, and more accurate. Whether you’re a beginner or an experienced professional, the following materials can reinforce your knowledge and enhance your proficiency.
1. Online Conversion Calculators
The quickest way to get comfortable with pressure units is by using online pressure converters. These tools allow users to convert between the pascal in SI units and other common units like:
-
psi (pounds per square inch)
-
bar
-
mmHg (millimeters of mercury)
-
atm (atmospheres)
Recommended tools include:
-
NIST Unit Converter
-
EngineeringToolBox.com
-
UnitConverter.net
-
CalculatorSoup
Many of these platforms also offer mobile-friendly versions, making them useful for fieldwork or classroom use.
2. Educational Apps
Apps designed for engineering and physics students often feature built-in tools for pressure calculation and unit conversion. Some notable ones:
-
Wolfram Alpha: Allows symbolic and numeric calculations involving pressure in pascal in SI units.
-
Phyphox: Turns your smartphone into a pressure-sensing laboratory.
-
Pressure Converter by Intemodino (Android/iOS): Fast conversions with support for SI and non-SI units.
These apps offer intuitive interfaces and can serve as virtual learning assistants in technical training programs.
3. Interactive Simulations
Simulations help learners visualize how pressure works in real-time. They often allow users to manipulate force and area inputs to observe changes in pressure measured in pascal in SI units.
Popular platforms include:
-
PhET Interactive Simulations (by the University of Colorado Boulder)
-
SimScale (cloud-based engineering simulation)
-
COMSOL Multiphysics (for more advanced pressure modeling)
These tools are especially helpful in teaching students how pressure is distributed across surfaces or how it changes with volume and temperature.
4. Engineering Software and CAD Integration
Professional tools like ANSYS, SolidWorks, and AutoCAD Mechanical use the pascal in SI units as a default or optional pressure unit. Engineers can set boundary conditions, test simulations, and verify tolerances using kPa, MPa, or GPa values.
Learning how to navigate these platforms can reinforce practical understanding of SI units while improving design efficiency.
5. Reference Charts and Conversion Tables
For quick offline access, printable conversion charts are extremely valuable. These often include:
-
Pressure equivalence tables (Pa to psi, bar, atm, etc.)
-
SI prefix multipliers (kilo, mega, giga)
-
Real-world pressure examples using pascal in SI units
Including these in textbooks, lab manuals, or workplace safety posters ensures that all team members have instant access to critical data.
6. Textbooks and Academic Resources
Foundational learning about the pascal in SI units can be built through well-structured educational materials, such as:
-
“Engineering Fundamentals and Problem Solving” by Eide et al.
-
“Fundamentals of Thermodynamics” by Sonntag and Borgnakke
-
NIST and ISO Guidelines on Pressure Measurement
These texts explain both theory and practical applications, often with step-by-step problem-solving approaches involving the pascal.
7. YouTube Channels and Tutorials
Visual learners can benefit from high-quality video tutorials that explain how to use the pascal in SI units in real-life scenarios. Recommended content includes:
-
Engineering Explained (automotive and pressure systems)
-
Khan Academy (introductory physics)
-
LearnChemE (engineering modules from the University of Colorado)
These resources help demystify complex pressure concepts using animations, analogies, and worked examples.
8. Hands-On Classroom Demonstrations
For instructors, classroom demos using syringes, balloons, or water columns can vividly illustrate pressure in terms of force per unit area. Measuring the resulting values in pascal in SI units helps students make the connection between math and physical experience.
Example: Use a bathroom scale and a small board to measure pressure as someone presses down with their hand, then convert the result to pascals using area and force estimates.
Summary and Conclusion
The pascal in SI units stands as a cornerstone of modern pressure measurement — a derived unit that provides simplicity, consistency, and universality in science, engineering, and industry. Named after Blaise Pascal, this unit represents one newton of force applied over one square meter, or 1 Pa = 1 N/m². Though seemingly small in scale, the pascal is used across a vast range of magnitudes, from atmospheric pressure measured in hectopascals to structural testing values expressed in megapascals or even gigapascals.
Throughout this article, we explored how the pascal in SI units is:
-
Defined within the SI framework, grounded in base units like kilogram, meter, and second.
-
Historically derived from the contributions of Blaise Pascal and the global movement toward standardized units.
-
Widely used in fields such as engineering, meteorology, medicine, aerospace, and scientific research.
-
Essential for safe design, testing, and reporting across multiple industries.
-
Easily convertible to and from other pressure units like psi, atm, bar, and mmHg using simple formulas.
-
Visualized through practical examples and comparisons to real-world forces.
-
Integrated into standards by ISO, ASME, and other global organizations to promote interoperability.
-
Supported by a wealth of educational tools and learning resources — from online converters to simulations, apps, and textbooks.
However, working with the pascal in SI units does require awareness of certain challenges: understanding the small scale of a single pascal, avoiding conversion errors, differentiating between gauge and absolute pressure, and always checking unit consistency in scientific tools and engineering designs.
By using the pascal in SI units, you’re participating in a global measurement system that fosters clarity, reproducibility, and safety. Whether you’re a student learning pressure equations for the first time, or a professional calibrating a precision instrument, mastering the pascal ensures your work is built on a standard that’s trusted and recognized worldwide.
FAQ:
1. What is a pascal in SI units?
A pascal in SI units is the standard derived unit of pressure defined as one newton per square meter. It is written as Pa, and represents the amount of force applied per unit area. It is used globally across science and engineering disciplines.
2. Why is the pascal used in the SI unit system for pressure?
The pascal in SI units is used because it provides a coherent and logical way to express pressure using existing base units: kilograms, meters, and seconds. This makes pressure calculations compatible with other SI-based quantities like force, mass, and acceleration.
3. How do I convert pascal in SI units to psi or atm?
To convert pascal in SI units to psi (pounds per square inch), divide by 6,894.76. To convert to atm (atmospheres), divide by 101,325. For example:
-
101,325 Pa = 1 atm
-
68,947.6 Pa = 10 psi
4. Is pascal in SI units used in everyday life?
Yes, the pascal in SI units is used in many everyday applications, though you might not realize it. Tire pressure, weather forecasts (in hectopascals), and even smartphone barometers all rely on pascals for accurate pressure readings.
5. What are kilopascals (kPa) and megapascals (MPa)?
Since one pascal in SI units is quite small, larger units are commonly used:
-
1 kPa = 1,000 Pa
-
1 MPa = 1,000,000 Pa
These are used in construction, hydraulics, and industrial applications.
6. Can I use pascal in SI units to measure vacuum pressure?
Absolutely. The pascal in SI units is often used in low-pressure and vacuum systems. In cleanrooms or semiconductor environments, pressure may be as low as a few pascals or even fractions of a pascal.
7. Who was Blaise Pascal and why is the unit named after him?
Blaise Pascal was a 17th-century French mathematician and physicist who conducted pioneering experiments in fluid mechanics and atmospheric pressure. The pascal in SI units honors his contributions to understanding pressure.
8. Is the pascal used in global technical standards?
Yes. The pascal in SI units is mandated by organizations like ISO, ASME, and IEC in technical specifications, product testing, and regulatory compliance documents worldwide.
9. How can students learn more about the pascal in SI units?
Students can explore the pascal in SI units using:
-
Online pressure calculators
-
Physics and engineering textbooks
-
Simulations (like PhET)
-
Mobile apps
These resources make the unit easier to visualize and apply.
10. What is the difference between gauge and absolute pressure in pascal in SI units?
Gauge pressure is measured relative to atmospheric pressure, while absolute pressure is measured relative to a vacuum. Both use pascal in SI units, but it’s critical to specify which type you’re using, especially in engineering and science applications.
Viscosity Units: Understanding and Converting Viscosity Measurements
Volumetric Flow Rate Units: Measurement, Conversion & Applications