In a world where science, engineering, commerce, and daily life intersect more than ever, having a unified system of measurement is crucial. This is where SI units—the International System of Units—come into play. Developed to standardize measurements across countries, disciplines, and industries, SI units provide a coherent framework that allows for universal communication, accuracy in scientific discovery, and efficiency in global trade.
Imagine trying to conduct international research or manufacture parts across different countries using incompatible units like inches, feet, pounds, or gallons. The margin for error would be enormous. With SI units, however, we benefit from a consistent language of measurement based on well-defined physical constants and universally accepted standards.
The concept of SI units goes beyond just metric units; it encompasses a logical structure of base and derived units, prefixes for scaling, and globally agreed-upon definitions. Whether you’re calculating energy in joules, measuring mass in kilograms, or timing events in seconds, you’re likely already using SI units—perhaps without even realizing it.
In this comprehensive guide, we will explore the origins, structure, importance, and applications of SI units. From the historical need for standardization to the modern redefinition of fundamental quantities using quantum constants, we’ll uncover why SI units are not just important but essential in today’s interconnected world. Whether you’re a student, an engineer, a teacher, or simply curious, this article aims to provide everything you need to understand what SI units are—and why they matter.
1.What are SI units?
SI units are the International System of Units (Système International d’Unités in French), which is the globally standardized system used to measure physical quantities like length, mass, time, temperature, electric current, and more. It is based on seven base units, from which all other units (called derived units) are formed.
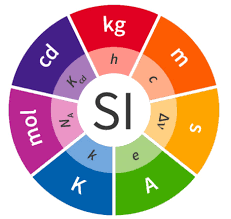
✅ The Seven Base SI Units:
| Quantity | Unit Name | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
✅ Key Features of SI Units:
-
Universal: Used worldwide in science, engineering, and most industries
-
Consistent: Based on natural constants (e.g., speed of light, Planck constant)
-
Scalable: Uses prefixes like milli-, kilo-, mega- for large/small values
-
Coherent: All derived units relate logically to base units
✅ Why Are SI Units Important?
-
They eliminate confusion in international collaboration
-
They improve accuracy in scientific research and manufacturing
-
They simplify learning by providing a single, consistent system
In short, SI units are the official, scientific language of measurement, ensuring everyone measures and communicates quantities in the same way across the globe.
2. The Origin and Evolution of SI Units
The need for standardized measurement systems has existed for thousands of years. Early civilizations, from the Egyptians to the Romans, created their own measurement systems, often based on body parts or arbitrary standards—such as the length of a foot or the width of a hand. These systems, while useful locally, lacked consistency and universality, which became a major issue as trade and scientific inquiry expanded across borders.
The Birth of the Metric System
The roots of SI units trace back to the French Revolution in the late 18th century. At that time, France had hundreds of regional units of measure, creating confusion and inefficiency. In response, the French Academy of Sciences developed a system based on natural, invariable quantities—thus the metric system was born in 1795. It introduced units such as the meter (based on the Earth’s meridian), the kilogram (based on a liter of water), and the second (based on astronomical time).
The Metre Convention of 1875
As the scientific community realized the value of standardization, 17 nations signed the Metre Convention in 1875. This landmark agreement led to the creation of the International Bureau of Weights and Measures (BIPM), tasked with maintaining international measurement standards. It also paved the way for the evolution of the metric system into a more formalized and globally recognized structure.
Creation of the SI System in 1960
The term SI units officially came into existence in 1960 during the 11th General Conference on Weights and Measures (CGPM). SI stands for Système International d’Unités, or the International System of Units. It built upon the metric system but offered greater structure, introducing a coherent set of base and derived units, along with defined prefixes and rules for use in science and industry.
Modernization and Redefinitions
While early SI units were based on physical objects (like the platinum-iridium kilogram prototype), this changed in the 21st century. In 2019, the CGPM approved a quantum redefinition of four base units: the kilogram, ampere, kelvin, and mole. These units are now defined using fundamental physical constants such as the Planck constant and the speed of light, ensuring that SI units are stable and reproducible across time and space, anywhere in the universe.
A Truly Global System
Today, nearly every country in the world uses SI units as the primary or official system of measurement. Even countries like the United States, which still use customary units in everyday life, rely on SI units for science, medicine, and global trade. From scientific publications to product specifications, SI units form the backbone of modern measurement.
3. Structure of SI Units
The SI units system is carefully structured to provide clarity, consistency, and universal applicability. It is built upon two main components: base units and derived units. This structure allows scientists, engineers, and everyday users to express any measurable quantity accurately and logically.
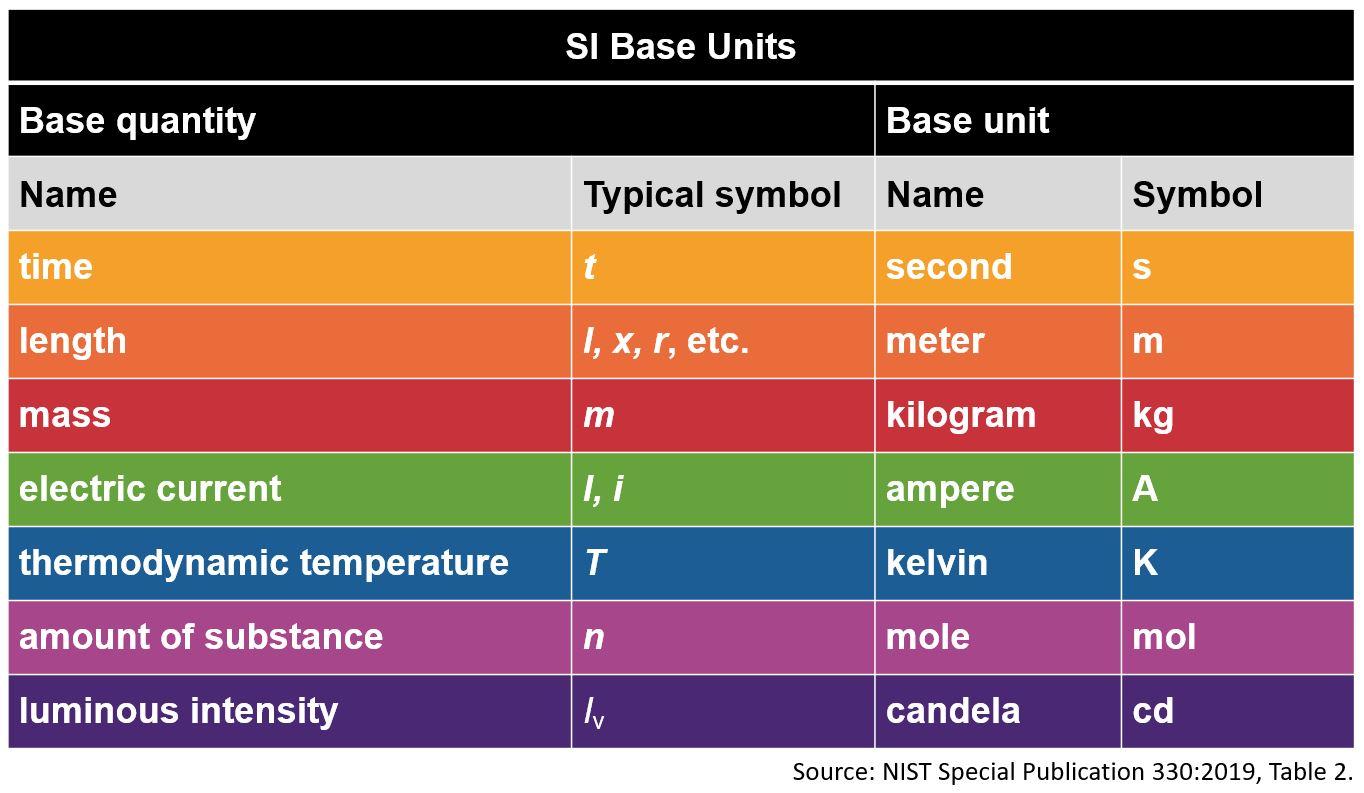
3.1 The Seven Base SI Units
At the heart of the International System of Units are seven base units, each representing a fundamental physical quantity. These base units serve as the foundation upon which all other SI units are built.
| Quantity | Unit Name | Unit Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Thermodynamic temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
Each of these units is precisely defined using natural constants to ensure maximum accuracy and reproducibility.
● Meter (m)
Defined as the distance light travels in a vacuum in 1/299,792,458 of a second.
● Kilogram (kg)
Defined using the Planck constant, which is fixed at exactly 6.62607015 × 10⁻³⁴ J·s.
● Second (s)
Defined as the duration of 9,192,631,770 periods of the radiation corresponding to the transition between two energy levels of the cesium-133 atom.
● Ampere (A)
Defined using the elementary charge, fixed at exactly 1.602176634 × 10⁻¹⁹ coulombs.
● Kelvin (K)
Defined by fixing the value of the Boltzmann constant at 1.380649 × 10⁻²³ J/K.
● Mole (mol)
Defined by fixing the Avogadro constant at 6.02214076 × 10²³ entities per mole.
● Candela (cd)
Defined using the luminous efficacy of monochromatic radiation of frequency 540 × 10¹² Hz.
3.2 Derived SI Units
Derived units are formed by combining the base units in algebraic expressions to measure more complex physical properties such as force, pressure, energy, and voltage.
Examples of derived SI units:
| Quantity | Unit Name | Unit Symbol | Base Unit Expression |
|---|---|---|---|
| Force | newton | N | kg·m/s² |
| Pressure | pascal | Pa | N/m² = kg/m·s² |
| Energy | joule | J | N·m = kg·m²/s² |
| Power | watt | W | J/s = kg·m²/s³ |
| Electric charge | coulomb | C | A·s |
| Voltage | volt | V | W/A = kg·m²/s³·A |
| Frequency | hertz | Hz | 1/s |
These derived SI units maintain consistency because they are based on combinations of the original seven base units.
3.3 Supplementary Units and Dimensionless Quantities
Some units in the SI framework are dimensionless, yet still extremely important. For example:
-
Radian (rad) – measures plane angles
-
Steradian (sr) – measures solid angles
Although not considered base units, they are coherent with SI units and are derived logically from the base set.
3.4 The Coherence of SI Units
One of the greatest strengths of the SI units system is coherence. This means that no conversion factors are needed when combining or manipulating quantities expressed in SI. This eliminates confusion and simplifies calculations in physics, chemistry, and engineering.
4. Derived SI Units and Their Applications
While the seven base units of the International System of Units form the foundation, it is the derived SI units that allow us to measure the full range of physical phenomena encountered in science, engineering, and everyday life. These units are created by algebraically combining base units to express complex quantities such as force, energy, and pressure.
4.1 What Are Derived SI Units?
Derived SI units are units formed by multiplying or dividing base units without the need for any conversion factor. This maintains the coherence of the SI system and allows for precise and consistent calculations.
For example:
-
The unit of force (newton) is defined as:
1 N = 1 kg·m/s² -
The unit of energy (joule) is:
1 J = 1 N·m = 1 kg·m²/s²
4.2 Commonly Used Derived SI Units
Below is a table of frequently used derived SI units:
| Physical Quantity | Derived Unit Name | Unit Symbol | Base Unit Expression |
|---|---|---|---|
| Force | newton | N | kg·m/s² |
| Pressure | pascal | Pa | N/m² = kg/m·s² |
| Energy | joule | J | N·m = kg·m²/s² |
| Power | watt | W | J/s = kg·m²/s³ |
| Electric charge | coulomb | C | A·s |
| Voltage | volt | V | W/A = kg·m²/s³·A |
| Capacitance | farad | F | C/V = s⁴·A²/kg·m² |
| Resistance | ohm | Ω | V/A = kg·m²/s³·A² |
| Conductance | siemens | S | A/V = s³·A²/kg·m² |
| Magnetic flux | weber | Wb | V·s = kg·m²/s²·A |
| Magnetic field strength | tesla | T | Wb/m² = kg/s²·A |
| Frequency | hertz | Hz | 1/s |
| Illuminance | lux | lx | lm/m² = cd·sr/m² |
| Radioactivity | becquerel | Bq | 1/s |
4.3 Practical Applications in Science and Industry
The use of SI units ensures that professionals across disciplines can work together without miscommunication or conversion errors.
● Physics and Engineering
-
Engineers calculate force using newtons (N), energy using joules (J), and power using watts (W).
-
Electrical engineers use volts (V), ohms (Ω), and amperes (A) for circuit design and analysis.
● Medicine and Biology
-
The gray (Gy) and sievert (Sv)—both derived SI units—are used to measure radiation dosage.
-
Concentrations of substances are often reported in moles per liter, another derived unit.
● Environmental Science
-
Pressure is reported in pascals (Pa) when analyzing atmospheric conditions.
-
Energy consumption and production are tracked in joules (J) or megajoules (MJ).
● Everyday Life
-
Home electricity usage is measured in kilowatt-hours (kWh), a practical unit derived from watts.
-
Speakers are rated in watts to indicate output power.
4.4 Advantages of Derived SI Units
-
Universality: The same units are used across countries and languages.
-
Consistency: All units derive from the same foundational principles.
-
Simplicity: Coherent units eliminate the need for extra conversion factors.
4.5 SI Units in Scientific Research
Nearly all scientific journals and international research standards require the use of SI units. This makes it easier to compare experimental results, replicate studies, and establish universal constants. Derived SI units are central to modeling everything from quantum mechanics to space exploration.
5. SI Unit Prefixes
One of the strengths of the SI units system is its scalability. By adding standardized prefixes to base and derived units, we can represent both extremely large and extremely small quantities without ambiguity. These SI unit prefixes make communication more efficient and calculations more manageable across various scientific and industrial domains.
5.1 What Are SI Unit Prefixes?
SI unit prefixes are symbols added before the unit to indicate multiplication or division by a power of ten. For example:
-
1 kilometer (km) = 1,000 meters
-
1 milligram (mg) = 0.001 grams
They help express values that would otherwise be too large or too small using just base units.
5.2 Table of Common SI Unit Prefixes
Below is a list of the most commonly used SI unit prefixes, ranging from the largest to the smallest:
| Prefix | Symbol | Factor | Power of 10 |
|---|---|---|---|
| yotta | Y | 1,000,000,000,000,000,000,000,000 | 10²⁴ |
| zetta | Z | 1,000,000,000,000,000,000,000 | 10²¹ |
| exa | E | 1,000,000,000,000,000,000 | 10¹⁸ |
| peta | P | 1,000,000,000,000,000 | 10¹⁵ |
| tera | T | 1,000,000,000,000 | 10¹² |
| giga | G | 1,000,000,000 | 10⁹ |
| mega | M | 1,000,000 | 10⁶ |
| kilo | k | 1,000 | 10³ |
| hecto | h | 100 | 10² |
| deca | da | 10 | 10¹ |
| (base unit) | 1 | 10⁰ | |
| deci | d | 0.1 | 10⁻¹ |
| centi | c | 0.01 | 10⁻² |
| milli | m | 0.001 | 10⁻³ |
| micro | µ | 0.000001 | 10⁻⁶ |
| nano | n | 0.000000001 | 10⁻⁹ |
| pico | p | 0.000000000001 | 10⁻¹² |
| femto | f | 0.000000000000001 | 10⁻¹⁵ |
| atto | a | 0.000000000000000001 | 10⁻¹⁸ |
| zepto | z | 0.000000000000000000001 | 10⁻²¹ |
| yocto | y | 0.000000000000000000000001 | 10⁻²⁴ |
5.3 New SI Prefixes (2022 Update)
In 2022, the CGPM added new prefixes to accommodate extremely large and small measurements:
| Prefix | Symbol | Factor | Power of 10 |
|---|---|---|---|
| ronna | R | 10²⁷ | 1,000 ronnameters = 1×10²⁷ m |
| quetta | Q | 10³⁰ | 1 quettabyte = 1×10³⁰ bytes |
| ronto | r | 10⁻²⁷ | 1 rontogram = 1×10⁻²⁷ g |
| quecto | q | 10⁻³⁰ | 1 quectometer = 1×10⁻³⁰ m |
These were introduced primarily to support big data, astrophysics, and quantum computing fields.
5.4 Practical Applications of SI Prefixes
● Technology
-
Computer memory: kilobytes (kB), megabytes (MB), gigabytes (GB)
-
Data transfer: megabits per second (Mbps)
-
Battery storage: kilowatt-hours (kWh)
● Science & Engineering
-
Distances: nanometers (nm) in molecular biology and semiconductor fabrication
-
Time: microseconds (µs) in signal processing
-
Mass: milligrams (mg) in pharmaceuticals
● Daily Use
-
Road signs: kilometers (km) for distance
-
Food labels: grams (g) and milliliters (mL) for portion sizes
5.5 Benefits of Using SI Unit Prefixes
-
Scalability: Allows for the measurement of vastly different magnitudes with ease
-
Clarity: Reduces the risk of misinterpreting large or small numbers
-
Consistency: Keeps values within a manageable range, improving readability
6. Benefits of Using SI Units
The adoption of SI units has transformed global communication, commerce, science, and engineering by providing a standardized, consistent, and universal system of measurement. From simplifying academic learning to improving international trade, the advantages of using SI units are vast and far-reaching.
6.1 Global Standardization
One of the most significant benefits of SI units is the establishment of a universal language of measurement. Regardless of location, industry, or language, professionals across the world can communicate data, specifications, and results without confusion or conversion errors.
Examples:
-
A scientist in Japan and an engineer in Germany can both reference a distance in meters (m) without ambiguity.
-
International product specifications list dimensions in millimeters (mm), ensuring uniformity in design and manufacturing.
6.2 Improved Accuracy and Precision
Because SI units are based on unchanging natural constants (like the speed of light or Planck constant), they offer high precision and reproducibility. This is essential in scientific research, pharmaceuticals, aerospace engineering, and any field that demands extremely accurate measurements.
Key Points:
-
Definitions of units like the kilogram and second are now tied to quantum constants, not physical artifacts.
-
Reproducible anywhere in the world using fundamental physical laws.
6.3 Simplified Calculations and Coherence
The coherent structure of the SI system eliminates the need for cumbersome conversion factors. Units naturally combine using algebraic rules, which simplifies formulas and promotes error-free calculations.
For example:
-
Power = Energy / Time →
Watt = Joule / Second = kg·m²/s³(no extra constants needed)
6.4 Enhanced Educational Value
SI units are widely taught in schools and universities because they are logical, easy to learn, and consistent. Students only need to learn a few base units and how to use prefixes to measure everything from atomic scales to cosmic distances.
-
Supports STEM education globally
-
Builds foundational understanding for advanced topics in physics, chemistry, and engineering
6.5 Cost and Time Efficiency in Industry
Using SI units across an entire organization or industry streamlines design, testing, logistics, and documentation. This reduces:
-
Time spent on conversions
-
Risk of costly errors (e.g., incorrect dimensions, incompatible parts)
-
Regulatory discrepancies in global markets
6.6 Legal and Regulatory Compliance
Most international standards organizations (ISO, IEC, ASTM) and national laws require or strongly recommend using SI units. Products not specified in SI may face import/export restrictions or require costly re-labeling.
-
Ensures compliance with product labeling laws
-
Meets the requirements of technical documentation in international contracts
Using SI units enhances consistency, safety, and efficiency across all sectors. Whether you’re building rockets, designing pharmaceuticals, or studying weather patterns, SI units offer the clarity and reliability essential for success.
7. Common Mistakes and Misconceptions About SI Units
Despite the widespread adoption and clarity of the SI units system, mistakes and misconceptions still occur—both in education and professional practice. These errors can lead to misinterpretation of data, flawed calculations, and even costly failures in engineering and scientific research.
7.1 Confusing SI Units with Non-SI Units
One of the most frequent mistakes is assuming that commonly used units in everyday life are part of the SI system when they are not.
Examples:
-
Pound (lb) is not an SI unit; kilogram (kg) is.
-
Inch (in) is not SI; meter (m) or millimeter (mm) is.
-
Fahrenheit (°F) is not SI; kelvin (K) or Celsius (°C) is used in SI-compatible contexts.
This confusion often occurs in countries where customary systems, such as the Imperial or U.S. customary system, are still in use alongside SI.
7.2 Misuse of Unit Symbols and Capitalization
SI unit symbols are case-sensitive and follow strict formatting rules. Incorrect usage can lead to ambiguity or miscommunication.
Common Symbol Mistakes:
| Incorrect | Correct | Explanation |
|---|---|---|
| Sec | s | “Second” is always written as lowercase “s” |
| Kg | kg | Unit symbols never begin with a capital |
| M/S | m/s | Use lowercase and proper slash placement |
| Watt | W | “Watt” is capitalized because it’s a proper name |
7.3 Mixing Unit Systems
Combining SI units with non-SI units in calculations can lead to major errors if not carefully converted. This is especially problematic in engineering and construction projects.
Real-world Example:
NASA’s Mars Climate Orbiter failed in 1999 due to a unit mismatch: one team used pound-seconds, while the navigation software expected newton-seconds—both units of force, but from different systems.
7.4 Incorrect Use of Prefixes
SI prefixes must be used correctly, with no space between the prefix and the unit, and no combination of prefixes.
Errors to Avoid:
-
Writing “m kilo grams” instead of “mg” or “kg”
-
Stacking prefixes like “microkilogram” (not allowed; use appropriate scaling)
7.5 Misinterpreting Decimal Points and Grouping
Different regions use different decimal separators (comma vs. period), but SI guidelines recommend using the period (.) as the decimal marker, and a space—not a comma—to separate groups of three digits.
Recommended Format:
-
Correct: 12 345.678
-
Avoid: 12,345.678 (comma can be misread as a decimal)
7.6 Believing SI Units Are Just the Metric System
While SI units evolved from the metric system, they are far more structured. SI includes not only base units and decimals but also derived units, coherent prefixes, and precise definitions based on fundamental constants.
Understanding and correctly using SI units helps ensure clear communication, scientific accuracy, and professional credibility. Avoiding these common pitfalls reinforces the integrity of your data, calculations, and conclusions.
8. SI Units vs. Other Unit Systems
While the SI units system is the international standard, other unit systems such as the Imperial system and U.S. customary units are still in use in various countries. Understanding the differences between these systems—and the advantages of SI units—can help avoid costly mistakes and improve clarity in global communication.
8.1 Commonly Used Unit Systems
● SI Units (International System of Units)
-
Origin: Evolved from the metric system
-
Global adoption: Used officially by nearly every country in science, education, and industry
-
Structure: Based on seven base units and a set of coherent derived units
-
Use of prefixes for scaling: kilo-, milli-, micro-, etc.
● Imperial System
-
Origin: British Empire
-
Units: Inch, foot, yard, mile (length); pound, ounce, stone (mass); gallon, pint (volume)
-
Current use: Official in only a few sectors in the UK
● U.S. Customary Units
-
Origin: Derived from the Imperial system
-
Units: Similar to Imperial, but with variations (e.g., fluid ounces, gallons differ in volume)
-
Current use: Predominantly used in the United States for non-scientific purposes
8.2 Side-by-Side Comparison Table
| Measurement | SI Units | Imperial / U.S. Units |
|---|---|---|
| Length | meter (m) | inch (in), foot (ft), mile |
| Mass | kilogram (kg) | pound (lb), ounce (oz) |
| Temperature | kelvin (K), Celsius (°C) | Fahrenheit (°F) |
| Volume | liter (L) (non-SI accepted) | gallon, pint, quart |
| Force | newton (N) | pound-force (lbf) |
| Pressure | pascal (Pa) | psi (pounds per square inch) |
| Energy | joule (J) | BTU, foot-pound |
8.3 Problems with Using Non-SI Units
● Inconsistency
Non-SI systems are often not coherent, meaning calculations may require cumbersome conversion factors.
● Global Confusion
Measurements in different units often need conversion, which can lead to errors—especially in international contracts, scientific publications, or manufacturing.
● Education Barriers
Learning two or more unit systems can be confusing for students, slowing down STEM education and global collaboration.
8.4 Historical and Cultural Usage
Some countries continue using customary systems due to historical, cultural, or practical reasons. For instance:
-
The United States still uses miles, pounds, and Fahrenheit in daily life.
-
The UK uses a mix of Imperial and SI (e.g., miles on road signs but kilograms for food labels).
However, in science, medicine, and industry, SI units are almost universally preferred for their clarity, scalability, and consistency.
8.5 Advantages of SI Units Over Other Systems
| Advantage | SI Units |
|---|---|
| Based on physical constants | ✅ |
| Coherent structure | ✅ |
| Scalable using prefixes | ✅ |
| Universally adopted in science | ✅ |
| Requires fewer conversions | ✅ |
8.6 Transition to SI Units
Countries that still rely heavily on customary systems are making gradual transitions:
-
Industries like aerospace, electronics, and medicine in the U.S. use SI units exclusively.
-
Scientific research papers must report results in SI, regardless of the author’s country
While Imperial and U.S. customary systems persist in some places, SI units dominate in science, trade, engineering, and education due to their logical structure, ease of use, and international consistency. Adopting SI units helps bridge global gaps in understanding and collaboration.
9. Modern Redefinitions and Quantum Standards
The SI units system is not static. It has evolved to reflect advances in science and technology. One of the most significant shifts in the history of SI units occurred in 2019, when four of the seven base units were redefined based on fundamental physical constants. These redefinitions marked a new era of quantum-based measurement, ensuring greater precision and universality.
9.1 Why Redefine SI Units?
Historically, some base SI units were defined using physical artifacts. For example, the kilogram was once based on a platinum-iridium cylinder stored in France. However, reliance on physical objects introduces problems:
-
Objects can change mass over time due to contamination or wear.
-
Access to the standard is geographically limited.
-
It’s not possible to perfectly replicate or verify physical artifacts globally.
To overcome these limitations, scientists moved toward definitions based on unchanging constants of nature, such as the speed of light and Planck’s constant.
9.2 The 2019 Redefinition: A Quantum Leap
In May 2019, the General Conference on Weights and Measures (CGPM) approved a revision of the SI that redefined four base units:
-
Kilogram (kg)
-
Ampere (A)
-
Kelvin (K)
-
Mole (mol)
These are now tied to the following fundamental constants:
| SI Unit | Now Defined Using | Constant Value |
|---|---|---|
| Kilogram | Planck constant (h) | 6.62607015 × 10⁻³⁴ J·s |
| Ampere | Elementary charge (e) | 1.602176634 × 10⁻¹⁹ C |
| Kelvin | Boltzmann constant (k) | 1.380649 × 10⁻²³ J/K |
| Mole | Avogadro constant (Nₐ) | 6.02214076 × 10²³ mol⁻¹ |
9.3 Benefits of Quantum-Based SI Units
● Universality
These constants are the same everywhere in the universe, making SI units truly global and timeless.
● Precision
Measurement uncertainty is drastically reduced. Scientists can now replicate base units with ultra-high precision using advanced instruments.
● Independence from Artifacts
There is no longer a need to maintain or protect a physical object as the definition of a unit. The units are now replicable in any advanced laboratory.
● Accessibility
With the right equipment, any national metrology institute can realize SI units independently, enabling better worldwide measurement equality.
9.4 Impact on Metrology and Technology
The redefinition has enabled:
-
More accurate calibration of instruments used in quantum computing, semiconductors, and nanotechnology
-
High-precision timekeeping, essential for GPS, telecommunications, and scientific research
-
Consistent and reproducible measurements for drug formulation, climate science, and advanced physics
9.5 Continuity and Backward Compatibility
Despite the redefinitions, the values of the units themselves have not changed in practice. For users, 1 kilogram is still 1 kilogram, but it is now tied to an unchanging constant rather than a fragile artifact. This ensures full compatibility with previous standards and industrial systems.
The modernization of SI units through quantum standards represents a significant scientific achievement. By anchoring measurements in the constants of nature, the world now has a measurement system that is more
10. SI Units in Education and Industry
The widespread implementation of SI units across educational institutions and industries plays a crucial role in creating a unified, scientifically literate, and globally connected society. Their structured, logical, and scalable nature makes SI units the cornerstone of modern learning and technical application.
10.1 SI Units in Education
From early schooling to advanced university degrees, SI units are introduced as the standard system of measurement in science, technology, engineering, and mathematics (STEM). This early exposure helps students:
-
Develop a consistent understanding of measurement
-
Perform unit-based problem solving with ease
-
Transition smoothly into technical fields and research roles
Applications in the Classroom:
-
Physics classes use meters, seconds, and newtons for mechanics
-
Chemistry students work with moles, liters, and kelvins
-
Biology courses adopt grams and micrometers for cellular study
Standardizing with SI units ensures that learners across different countries are aligned with the same measurement system, promoting easier collaboration and academic mobility.
10.2 SI Units in Engineering and Manufacturing
In fields like mechanical, civil, electrical, and chemical engineering, SI units are vital for design, production, and quality assurance.
Examples:
-
Mechanical Engineers use newtons (N) for force, joules (J) for energy
-
Electrical Engineers rely on volts (V), amperes (A), and watts (W)
-
Civil Engineers design infrastructure with meters (m) and pascals (Pa) for structural loads
Using SI units eliminates the need for conversions, which can introduce errors and delays in complex calculations or safety-critical systems.
10.3 SI Units in Healthcare and Pharmaceuticals
In medicine and health sciences, SI units improve dosage accuracy, diagnostics, and global standardization in treatments.
Applications:
-
Drug dosages expressed in milligrams (mg) and micrograms (µg)
-
Body temperature measured in Celsius (°C) or Kelvin (K)
-
Blood tests report glucose or cholesterol in millimoles per liter (mmol/L)
SI-compliant data ensures consistent medical documentation and facilitates international research trials and drug approval processes.
10.4 SI Units in the Energy and Environmental Sector
Energy production, consumption, and environmental monitoring heavily rely on SI units to quantify impact and efficiency.
Examples:
-
Power plants report capacity in megawatts (MW)
-
Pollution levels measured in micrograms per cubic meter (µg/m³)
-
Solar panel outputs calculated in kilowatt-hours (kWh)
SI-based reporting supports global standards such as ISO 50001 (Energy Management) and UN climate agreements.
10.5 SI Units in Global Trade and Logistics
For international trade, consistent units are essential. SI units:
-
Appear on shipping manifests and safety data sheets
-
Define weight and dimensions in customs documentation
-
Enable smooth product certification and regulatory compliance
Industries that adhere to SI units can expand globally with minimal reconfiguration.
10.6 Industry Standards and Regulatory Bodies
International bodies such as ISO, IEC, ASTM, and WHO require the use of SI units in their documentation, labeling, and testing procedures.
Benefits:
-
Reduces confusion and compliance errors
-
Promotes cross-border collaboration
-
Builds trust with partners and customers
From the classroom to the factory floor, and from laboratories to global markets, SI units provide the backbone for measurement. Their universal adoption improves communication, safety, accuracy, and productivity in virtually every sector.
11. Conversion Between SI Units and Non-SI Units
Despite the global dominance of SI units, non-SI units like inches, pounds, gallons, and Fahrenheit are still commonly used in daily life, particularly in the United States and parts of the United Kingdom. Understanding how to convert between SI units and other systems is essential for global trade, scientific collaboration, and avoiding costly mistakes.
11.1 Why Conversion Matters
-
International Business: Product specifications must align across borders
-
Scientific Research: Results must be standardized in SI for publication
-
Engineering Design: Components sourced globally need consistent units
-
Daily Life: Recipes, body measurements, weather reports, and more
Mistakes in conversion can lead to miscommunication, financial loss, or even catastrophic failure in engineering projects.
11.2 General Guidelines for Conversion
-
Know the conversion factor
Example: 1 inch = 25.4 millimeters -
Ensure unit compatibility
Never convert between unrelated units (e.g., volume to length) -
Use the correct number of significant digits
Maintain precision based on the original measurement’s accuracy -
Use reliable tools or software
Especially for technical or large-scale conversions
11.3 Common Conversions from Non-SI to SI Units
| Non-SI Unit | SI Equivalent | Conversion Factor |
|---|---|---|
| Inch (in) | Meter (m) | 1 in = 0.0254 m |
| Foot (ft) | Meter (m) | 1 ft = 0.3048 m |
| Mile (mi) | Kilometer (km) | 1 mi = 1.60934 km |
| Pound (lb) | Kilogram (kg) | 1 lb = 0.453592 kg |
| Ounce (oz) | Gram (g) | 1 oz = 28.3495 g |
| Gallon (US) | Liter (L) | 1 gal = 3.78541 L |
| Fahrenheit (°F) | Celsius (°C) | (°F − 32) × 5⁄9 = °C |
| PSI | Pascal (Pa) | 1 psi = 6,894.76 Pa |
| BTU | Joule (J) | 1 BTU = 1,055.06 J |
11.4 Reverse Conversions (SI to Non-SI)
| SI Unit | Common Non-SI Equivalent | Conversion Formula |
|---|---|---|
| Meter (m) | Inch (in) | 1 m = 39.3701 in |
| Kilogram (kg) | Pound (lb) | 1 kg = 2.20462 lb |
| Liter (L) | Gallon (US) | 1 L = 0.264172 gal |
| Celsius (°C) | Fahrenheit (°F) | (°C × 9⁄5) + 32 = °F |
11.5 Conversion Tools and Calculators
For accuracy and ease, many professionals and students use:
-
Online unit converters
-
Mobile apps
-
Engineering software (AutoCAD, MATLAB, etc.)
-
Built-in calculator functions
When converting large quantities or critical measurements, using calibrated software tools ensures that the integrity of data is preserved.
11.6 Challenges in Conversion
-
Rounding Errors: Especially with repeated conversions
-
Dimensional Mistakes: Confusing mass vs. weight, or volume vs. area
-
Unit Symbol Confusion: E.g., “m” can mean meter or milli depending on context
To mitigate these challenges, always confirm:
-
The measurement context
-
The correct SI unit and prefix
-
Proper documentation and labeling
Mastering conversions between SI and non-SI units is a vital skill in today’s globalized world. Whether you’re designing products, analyzing data, or interpreting scientific results, accurate unit conversion ensures consistency, safety, and professionalism across all disciplines.
12. Future of SI Units
The journey of SI units from their metric origins to their current quantum-based definitions has been marked by continual improvement, driven by advances in science, technology, and global cooperation. Looking ahead, the future of SI units promises even more innovation as we respond to new demands from emerging fields like nanotechnology, quantum computing, and space exploration.
12.1 Continuous Improvement Through Science
The SI system is not fixed in time. It evolves as our understanding of the universe grows and as our ability to measure improves.
Key Trends:
-
More precise measurements using quantum technologies (e.g., atomic clocks)
-
Better realization of units in national metrology institutes through laser interferometry, Josephson junctions, and more
-
Deeper alignment with physical constants to enhance reproducibility worldwide
The system is governed by the International Committee for Weights and Measures (CIPM), which regularly reviews the definitions and applications of SI units.
12.2 Adapting to Emerging Scientific Fields
As technology continues to advance, SI units will support:
-
Nanoscience: Accurate measurements in nanometers (nm), femtoseconds (fs), and picojoules (pJ)
-
Quantum computing: SI-derived standards for quantum states, superposition, and entanglement
-
Biotechnology and medicine: Molecular-level precision in measurements, such as picomoles and attograms
These domains require extremely small and precise units, and the SI system—thanks to its scalability and structure—is well-suited to meet those needs.
12.3 Supporting the Digital Economy and Big Data
With the explosion of data in the modern world, SI units have recently expanded to include new prefixes:
| Prefix | Symbol | Factor |
|---|---|---|
| Ronna | R | 10²⁷ |
| Quetta | Q | 10³⁰ |
| Ronto | r | 10⁻²⁷ |
| Quecto | q | 10⁻³⁰ |
These prefixes are designed for:
-
Massive data storage (e.g., quettabytes)
-
Extremely small-scale measurements (e.g., rontograms)
-
Precision modeling of climate, finance, and social behavior at scale
12.4 SI Units in Space and Interplanetary Missions
As humanity expands its exploration of the solar system and beyond, SI units will remain central to:
-
Navigating planetary distances (e.g., gigameters, astronomical units)
-
Designing spacecraft components with micrometer tolerances
-
Coordinating interplanetary timekeeping systems based on atomic time
12.5 The Role of AI and Automation
Artificial intelligence (AI) and machine learning increasingly rely on standardized data, making SI units crucial for:
-
Interpretable and scalable algorithms
-
Accurate sensor data in automated systems
-
Industry 4.0 and smart manufacturing platforms
AI-driven systems also support automated unit conversion, unit checking, and error detection—further reducing human mistakes.
12.6 Global Policy and Education Support
To ensure equitable and consistent access to scientific tools, more countries are:
-
Investing in metrology education
-
Expanding adoption of SI units in schools and public infrastructure
-
Using SI-based policies in climate agreements, trade, and international law
The future of SI units lies in their flexibility and scientific integrity. As humanity ventures into new frontiers—whether technological, medical, or cosmic—SI units will continue to evolve to support discovery, innovation, and global understanding.
13. Conclusion
In a world that thrives on precision, consistency, and global connectivity, the SI units system stands as a pillar of modern civilization. From its historical roots in the French metric system to its current quantum-defined structure, SI units have become the universal language of measurement, unifying scientists, engineers, educators, and industries across every continent.
Throughout this article, we explored the full scope of what SI units are—from the foundational base units to the sophisticated derived quantities that define our understanding of force, energy, pressure, and beyond. We examined how SI prefixes enable scalability, how quantum redefinitions ensure scientific accuracy, and how conversion tools bridge the gap between SI and legacy systems.
The benefits of using SI units—standardization, coherence, accessibility, and precision—make them indispensable in a world where measurements govern everything from microchip design to climate modeling and from global trade to everyday health and nutrition.
Key Takeaways:
-
SI units are built on seven base units, with countless derived units enabling comprehensive measurement of physical quantities.
-
Their structure is coherent and scalable, supported by standardized prefixes for ease of use across large and small scales.
-
Recent redefinitions based on fundamental constants like the Planck constant and the speed of light ensure that SI units remain relevant and precise in the 21st century.
-
The adoption of SI units in education, industry, science, and policy promotes global consistency and minimizes costly errors.
-
As we enter a future of quantum technology, AI, and space exploration, SI units will continue to serve as the backbone of innovation and progress.
In essence, understanding what SI units are is not only a fundamental scientific literacy skill but also a practical necessity in our interconnected, data-driven world. Whether you’re a student, researcher, professional, or global citizen, SI units will continue to touch every part of your life—quietly, consistently, and powerfully.
14. FAQs About SI Units
To help reinforce your understanding and address some of the most common inquiries, here are frequently asked questions about SI units:
Q1: What are SI units?
SI units, or the International System of Units (Système International d’Unités), are the globally accepted standard for measuring physical quantities. The system consists of seven base units and a set of derived units built from them. SI units are used in science, engineering, commerce, education, and daily life around the world.
Q2: What is the difference between SI units and metric units?
The metric system is the predecessor of the SI system. While metric units like meters and grams are part of SI, SI units represent a more formal, structured, and internationally agreed-upon system that includes:
-
Defined base and derived units
-
Use of physical constants for definitions
-
Coherent rules for combining units and applying prefixes
In short, all SI units are metric, but not all metric units are SI.
Q3: Why are SI units important?
SI units offer:
-
Consistency in measurement across countries and disciplines
-
Accuracy and reproducibility based on natural constants
-
Ease of learning with logical scaling using prefixes
-
Global compatibility, crucial for international trade, research, and collaboration
Q4: Who defines SI units?
SI units are governed by:
-
The International Bureau of Weights and Measures (BIPM)
-
The General Conference on Weights and Measures (CGPM)
-
The International Committee for Weights and Measures (CIPM)
These organizations work together to ensure the integrity, evolution, and global adoption of the SI system.
Q5: Are SI units used everywhere in the world?
Yes, nearly all countries have adopted SI units as the official or primary measurement system—especially in science, education, and international trade. The United States, Liberia, and Myanmar still use customary units in daily life, but SI units are still used in scientific, medical, and industrial contexts in those countries.
Q6: What are the seven base SI units?
| Quantity | Unit Name | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
These serve as the building blocks for all other SI units.
Q7: Can SI units change over time?
Yes—but only in how they’re defined, not in value. For example, the kilogram used to be defined by a metal artifact but is now defined by the Planck constant. These changes are made to improve accuracy and global reproducibility and are backward-compatible.
In Summary:
Understanding SI units is essential for navigating the modern world. Whether you’re studying physics, building software, or importing goods, SI units provide the universal language of measurement—one that continues to evolve to meet the needs of science, technology, and humanity.