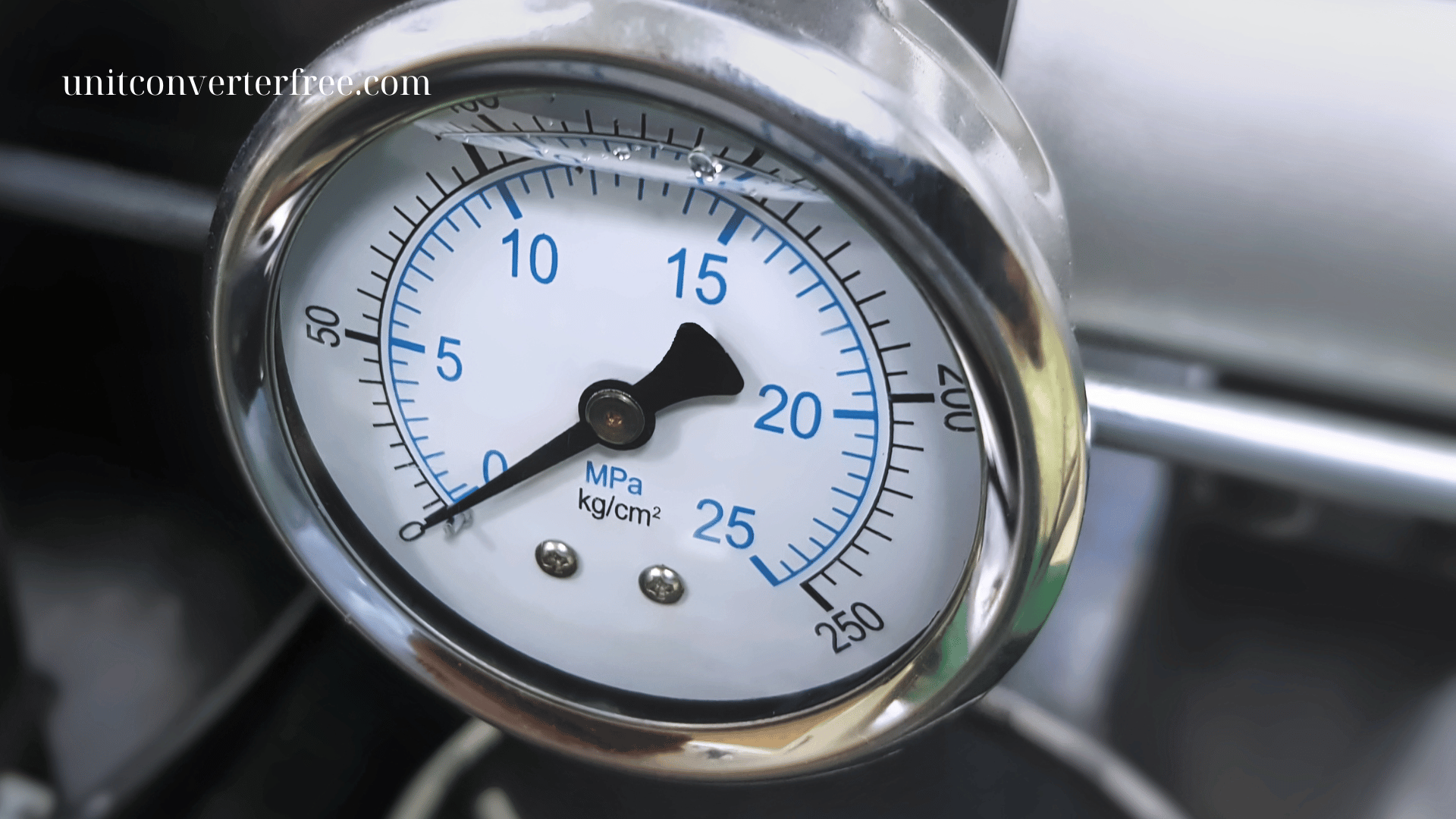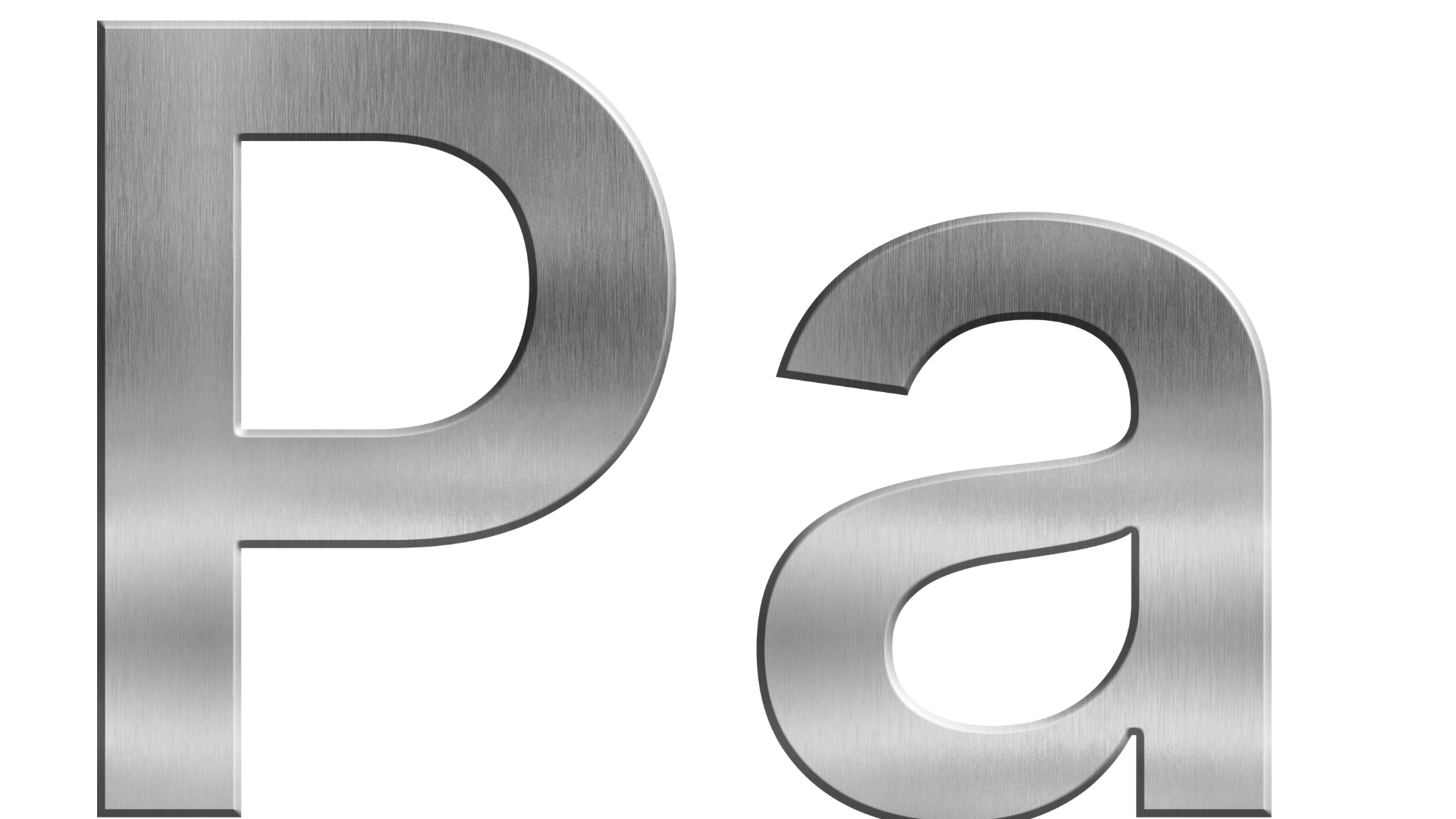Units of measurement are standardized quantities used to express and communicate numerical values of physical properties clearly and consistently. These units allow individuals, scientists, engineers, and industries worldwide to accurately measure, record, compare, and interpret data. Without standardized units, essential tasks like building infrastructure, conducting scientific experiments, trade, and even everyday activities like cooking or traveling would be confusing and prone to errors.
Throughout human history, the need for reliable units of measurement has driven civilizations to create diverse systems—from ancient units like the Egyptian cubit and the Roman mile, to the internationally recognized metric system adopted today. Modern society relies heavily on standardized units to ensure accuracy, efficiency, and effective communication across borders.
This article provides a comprehensive exploration of units of measurement, examining their historical evolution, types, applications, and significance across various fields. By understanding these units, readers will gain insight into their critical role in shaping our modern world and how standardized measurements continue to facilitate global collaboration, innovation, and progress.
What is a Unit of Measurement?
A unit of measurement is a defined quantity used as a standard to express and compare the magnitude of a physical quantity. It serves as a reference that allows people to describe, quantify, and communicate measurements consistently and accurately. Without units of measurement, numbers alone would lack meaning when describing length, weight, time, or any other physical property.
For example, saying an object is “5” means little unless we specify whether that “5” refers to meters, feet, kilograms, or seconds. The unit tells us what kind of quantity is being measured and the scale of that measurement.
Units of measurement can be:
-
Base Units: Fundamental units that define a single physical quantity, such as the meter for length or the second for time.
-
Derived Units: Combinations of base units, such as meters per second (m/s) for speed or newtons (N) for force.
Standardization of units of measurement ensures that everyone interprets measurements the same way, enabling clear communication in science, engineering, trade, and everyday life.
Historical Evolution of Units of Measurement
The concept of units of measurement dates back to ancient times when early civilizations needed practical ways to quantify length, weight, volume, and time for trade, construction, and daily life. These early units of measurement were often based on the human body or natural references, leading to many regional and inconsistent systems.
Ancient Civilizations and Their Units
-
Egyptian Cubit: One of the earliest known units, the cubit was based on the length of the forearm from the elbow to the tip of the middle finger. Ancient Egyptians used the cubit extensively in building monuments like the pyramids, showing early sophistication in measurement.
-
Babylonian and Mesopotamian Systems: These civilizations developed units for length, area, and volume primarily for agricultural and trade purposes. Their systems included measures such as the “kus” and “gur” for weight and capacity.
-
Roman Mile: The Romans standardized distance with the mile (“mille passus” meaning a thousand paces), which was roughly 1,000 double steps of a Roman soldier. This unit played a crucial role in mapping and building extensive road networks across the Roman Empire.
Medieval and Regional Variations
Throughout the Middle Ages, units of measurement remained largely localized, varying from town to town. This created confusion and inefficiencies in trade and construction. For example, the “foot” might differ in length depending on the region, and measures like the “stone” or “bushel” could have multiple definitions.
The Drive Toward Standardization
The Industrial Revolution and the expansion of international trade in the 18th and 19th centuries highlighted the need for consistent and universally accepted units of measurement. Scientists and governments began efforts to standardize units, which culminated in the creation of the metric system.
-
Metric System: Developed in France during the late 18th century, the metric system introduced units based on decimal relationships and natural constants, such as the meter defined by the Earth’s meridian. This system simplified conversions and made measurements more universal.
Modern Developments
Today, the International System of Units (SI) is the global standard, established in 1960 by the General Conference on Weights and Measures (CGPM). The SI system builds on the metric system and defines units based on fundamental physical constants, ensuring precision and universality across science, industry, and everyday use.
The historical evolution of units of measurement reflects humanity’s ongoing quest for accuracy, consistency, and communication. From body-based measurements to highly precise scientific definitions, this progression has been essential for technological advancement and global cooperation.
Categories of Units of Measurement
Units of measurement are organized into various categories based on the physical quantities they describe. Understanding these categories helps clarify how different aspects of the world are quantified and communicated. Below are the primary categories of units of measurement commonly used across science, engineering, and daily life:
1. Length
Measures the distance between two points. Examples include meters, feet, inches, and miles. Length units are essential in construction, navigation, and manufacturing.
2. Mass
Represents the amount of matter in an object. Units such as kilograms, grams, pounds, and ounces fall under this category. Mass is fundamental in fields like physics, chemistry, and commerce.
3. Time
Measures the duration or interval between events. Seconds, minutes, hours, and days are standard units of time, crucial for scheduling, scientific experiments, and technological processes.
4. Temperature
Describes the thermal state of a system or environment. Common units include Celsius, Fahrenheit, and Kelvin. Temperature measurements are vital in weather forecasting, engineering, and medicine.
5. Volume
Quantifies the amount of three-dimensional space an object or substance occupies. Liters, milliliters, gallons, and cubic meters are typical volume units used in cooking, shipping, and fluid dynamics.
6. Area
Measures the extent of a two-dimensional surface. Units such as square meters, acres, and hectares are used in land measurement, agriculture, and architecture.
7. Speed and Velocity
Indicates how fast an object is moving. Units like kilometers per hour, miles per hour, and meters per second are commonly used in transportation and physics.
8. Pressure
Measures force applied over a specific area. Units include pascals, bars, and pounds per square inch (psi). Pressure measurements are critical in meteorology, hydraulics, and automotive industries.
9. Energy and Power
Energy units such as joules and calories quantify work done or heat transferred, while power units like watts measure the rate of energy transfer. These units are important in physics, engineering, and electricity billing.
10. Electric Current and Voltage
Used to describe electrical quantities. Amperes measure current, volts measure electrical potential, and ohms measure resistance. These units are vital for electrical engineering and electronics.
Each category of units of measurement provides a framework to quantify specific physical properties, enabling precise communication, analysis, and application in a wide range of fields. Understanding these categories is essential for effectively using and converting units in practical and scientific contexts.
Measurement for Length
Length is one of the most fundamental physical quantities measured in various fields such as construction, engineering, navigation, and everyday activities. Units of measurement for length provide a standardized way to express distances, heights, depths, and sizes, allowing consistent communication and comparison.

Common Units of Length
-
Meter (m): The meter is the base unit of length in the International System of Units (SI). It was originally defined by the distance from the Earth’s equator to the North Pole but is now precisely defined by the speed of light. The meter is widely used around the world for scientific, industrial, and everyday measurements.
-
Kilometer (km): Equal to 1,000 meters, kilometers are typically used for measuring long distances such as those between cities or countries.
-
Centimeter (cm) and Millimeter (mm): These smaller units, where 1 meter = 100 centimeters and 1 centimeter = 10 millimeters, are used for more precise measurements, common in engineering, manufacturing, and tailoring.
Imperial Units
-
Inch, Foot, Yard, and Mile: Common in the United States and some other countries, these units have historical origins and remain in widespread use. For example:
-
1 foot = 12 inches
-
1 yard = 3 feet
-
1 mile = 5,280 feet
-
Historical Context
Before global standardization, units of length often varied locally. People used parts of the body such as the foot or the cubit (forearm length) as reference measures, which led to inconsistencies. The adoption of the metric system provided a uniform and decimal-based method for length measurement worldwide.
Applications
Length measurements are critical in fields like:
-
Construction: Accurate measurements ensure structural integrity and fit of components.
-
Manufacturing: Precise lengths are necessary for machining and assembly.
-
Navigation: Distances on maps and GPS systems use standardized units.
-
Everyday Life: Measuring height, fabric, or dimensions of furniture relies on familiar units of length.
Conversion Between Units
Understanding conversions between different units of measurement for length is important. For example:
-
1 inch = 2.54 centimeters
-
1 foot = 0.3048 meters
-
1 mile = 1.609 kilometers
Many tools and apps now simplify these conversions, but knowing the basics helps in interpreting and communicating length measurements effectively
Units of Measurement for Mass and Weight
Mass and weight are two related but distinct physical quantities, each measured using specific units of measurement essential in science, commerce, and everyday life.
Understanding Mass vs. Weight
-
Mass refers to the amount of matter in an object and is a fundamental property that does not change regardless of location.
-
Weight is the force exerted by gravity on that mass and can vary depending on gravitational pull (for example, on the Moon vs. Earth).
Because mass and weight are different concepts, their units of measurement also differ.
Common Units of Mass
-
Kilogram (kg): The kilogram is the base unit of mass in the International System of Units (SI). It is defined by the Planck constant, providing extreme precision for scientific purposes.
-
Gram (g) and Milligram (mg): Subunits of the kilogram, where 1 kilogram = 1,000 grams and 1 gram = 1,000 milligrams, are used for smaller quantities like food ingredients or pharmaceuticals.
-
Metric Ton (tonne): Equal to 1,000 kilograms, used for heavy materials and industrial measurements.
Imperial and US Customary Units of Weight
-
Pound (lb): Widely used in the United States, one pound is equivalent to approximately 0.4536 kilograms.
-
Ounce (oz): There are 16 ounces in a pound, commonly used for lighter items.
-
Ton: In the US, a ton usually refers to a short ton (2,000 pounds), while in the UK, a long ton equals 2,240 pounds.
Practical Applications
-
Science and Industry: Accurate mass measurements are crucial in chemistry, pharmaceuticals, and manufacturing to ensure quality and safety.
-
Commerce: Products like groceries are sold by weight, requiring standardized units for fair trade.
-
Everyday Use: People use scales at home to measure body weight or ingredients for cooking.
Conversion Examples
Understanding how to convert between units of mass and weight is important:
-
1 kilogram = 2.20462 pounds
-
1 pound = 16 ounces
-
1 metric ton = 1,000 kilograms = 2,204.62 pounds
Importance of Precision
In many fields, precise units of measurement for mass and weight are essential to avoid errors that could lead to safety hazards, financial losses, or scientific inaccuracies. Digital scales and calibrated instruments have helped increase measurement accuracy worldwide.
Units of Measurement for Time
Time is a fundamental dimension in the way humans perceive and organize their lives, activities, and the natural world. Measuring time accurately is crucial for everything from daily schedules to scientific research and technological processes. Units of measurement for time provide a consistent framework to quantify durations, intervals, and sequences of events.
Common Units of Time
-
Second (s): The second is the base unit of time in the International System of Units (SI). It is defined with extraordinary precision based on the vibrations of cesium atoms, making it the foundation for atomic clocks and global time standards.
-
Minute: Equal to 60 seconds, minutes are commonly used in everyday life to measure short periods, such as meeting durations or cooking times.
-
Hour: Comprising 60 minutes or 3,600 seconds, hours organize our day into manageable segments, regulating work, rest, and activities.
-
Day: Consisting of 24 hours, the day is based on the Earth’s rotation and forms the fundamental cycle of human life.
Larger Units of Time
-
Week: A grouping of 7 days, widely used for calendars and planning.
-
Month: Approximately 30 or 31 days (except February), months are based on the Moon’s orbit around Earth and are used for civil calendars.
-
Year: About 365.25 days, a year reflects Earth’s orbit around the Sun. Years are essential for tracking longer-term cycles such as seasons and aging.
Historical Context
Historically, humans have divided time based on natural phenomena like the Sun’s position (day and night) and the lunar cycle. Ancient civilizations developed calendars and devices like sundials and water clocks to measure time more precisely. The invention of mechanical clocks and, later, atomic clocks revolutionized the accuracy of time measurement.
Applications of Time Measurement
-
Daily Life: Scheduling, timekeeping, and coordinating activities depend on standardized time units.
-
Science and Technology: Experiments, satellite navigation, and telecommunications require precise time measurements.
-
Industry: Manufacturing processes and transportation systems rely on accurate timing for efficiency and safety.
Time Zones and Standardization
Global communication and travel require coordination of time across different regions. The introduction of time zones and Universal Coordinated Time (UTC) helps synchronize clocks worldwide, illustrating the importance of standardized units of measurement for time.
Units of Measurement for Temperature
Temperature is a key physical quantity that describes how hot or cold something is. The units of measurement for temperature are essential in science, engineering, weather forecasting, medicine, cooking, and many aspects of daily life. Standardized temperature units ensure clear communication and accurate data across different regions and industries.
Common Units of Measurement for Temperature
-
Celsius (°C): Also known as Centigrade, the Celsius scale is widely used around the world and in most scientific work. It sets the freezing point of water at 0°C and the boiling point at 100°C under standard atmospheric pressure.
-
Fahrenheit (°F): Commonly used in the United States for weather reports, cooking, and household thermometers. On the Fahrenheit scale, water freezes at 32°F and boils at 212°F.
-
Kelvin (K): The Kelvin scale is the SI base unit for temperature and is mainly used in science and engineering. It starts at absolute zero (0 K), the theoretical point where particles have minimum thermal motion. The size of one Kelvin is the same as one degree Celsius, but there are no negative numbers on the Kelvin scale.
Historical Context
The need to measure temperature precisely arose from scientific exploration and the development of thermometers. Early thermometers were based on the expansion of liquids like mercury or alcohol. The adoption of standardized temperature scales allowed for more accurate scientific research and industrial control.
Conversion Between Units
Understanding how to convert between different units of measurement for temperature is essential, especially when working internationally:
-
Celsius to Fahrenheit: °F = (°C × 9/5) + 32
-
Fahrenheit to Celsius: °C = (°F − 32) × 5/9
-
Celsius to Kelvin: K = °C + 273.15
Applications in Everyday Life
-
Weather: Temperature measurements help us prepare for daily activities and severe weather conditions.
-
Medicine: Body temperature readings are critical for diagnosing illnesses.
-
Industry: Manufacturing processes, chemical reactions, and food safety depend on accurate temperature control.
-
Science: Research in physics, chemistry, and environmental science requires precise and consistent temperature data.
Importance of Standardized Units
The use of clear, standardized units of measurement for temperature helps avoid confusion, particularly in international trade, scientific research, and global communication. Whether using Celsius, Fahrenheit, or Kelvin, knowing the context and conversion is crucial for accuracy.
Units of Measurement for Volume and Area
Understanding units of measurement for volume and area is essential in fields such as construction, agriculture, manufacturing, cooking, fluid dynamics, and land management. These units help quantify how much space an object occupies (volume) and the extent of a surface (area).
Units of Measurement for Volume
Volume is the amount of three-dimensional space a substance (solid, liquid, or gas) occupies.
Metric Units of Volume
-
Cubic meter (m³): The SI unit of volume; commonly used in large-scale engineering and industrial applications.
-
Liter (L): Widely used in daily life to measure liquids such as water, fuel, or beverages.
-
1 liter = 0.001 cubic meters (m³) = 1,000 milliliters (mL)
-
-
Milliliter (mL): A thousandth of a liter; used in medicine, cooking, and scientific measurements.
Imperial Units of Volume
-
Gallon (gal): Common in the US for measuring fuel and other liquids.
-
1 US gallon ≈ 3.785 liters
-
-
Quart, Pint, and Fluid Ounce: Subdivisions of a gallon used primarily in recipes and food products.
-
1 quart = 2 pints = 4 cups = 32 fluid ounces
-
Applications
-
Cooking: Recipes often specify volume in liters, cups, or tablespoons.
-
Science and Medicine: Precise liquid volumes are critical for experiments and dosages.
-
Industry: Fuel, chemicals, and manufacturing processes are managed using volume measurements.
Units of Measurement for Area
Area measures the extent of a two-dimensional surface or shape.
Metric Units of Area
-
Square meter (m²): The standard SI unit of area; used for flooring, room size, land plots.
-
Square centimeter (cm²) / Square millimeter (mm²): Used for small areas, such as in engineering or electronics.
-
Hectare (ha): Common in agriculture;
-
1 hectare = 10,000 square meters
-
Imperial Units of Area
-
Square foot (ft²): Common in real estate and construction.
-
Square inch (in²): Used for smaller items.
-
Acre: Standard land measurement in the US and UK;
-
1 acre = 43,560 square feet ≈ 4,047 square meters
-
Applications
-
Construction: Floor plans, wall areas, and site layout require accurate area calculations.
-
Real Estate: Property values and taxes are often based on land area.
-
Agriculture: Farmers use hectares or acres to manage land and crop planning.
Conversion Examples
Understanding conversions between different units of measurement for volume and area is vital:
-
1 m³ = 1,000 liters
-
1 ft² ≈ 0.0929 m²
-
1 acre ≈ 0.4047 hectares
-
1 cup (US) ≈ 237 mL
Units of Measurement for Speed and Velocity
Speed and velocity are key physical quantities that describe how fast something is moving. While speed measures the rate of motion regardless of direction, velocity also includes direction. The units of measurement for speed and velocity are essential in transportation, physics, meteorology, and sports.
Definition and Difference
-
Speed is the rate at which an object covers distance.
Formula: Speed = Distance ÷ Time -
Velocity is speed in a specified direction.
Formula: Velocity = Displacement ÷ Time
Though the concepts are similar, velocity is a vector quantity (has direction), while speed is scalar (has only magnitude).
Common Units of Measurement for Speed and Velocity
Metric Units
-
Meters per second (m/s): The SI unit for speed and velocity; widely used in scientific contexts.
-
Kilometers per hour (km/h): Commonly used in road signs, car speedometers, and daily activities.
-
1 km/h = 0.27778 m/s
-
-
Centimeters per second (cm/s): Used for very small-scale measurements, such as in laboratories or machinery.
Imperial Units
-
Miles per hour (mph): Standard unit used in the United States and the UK for road speeds.
-
Feet per second (ft/s): Used in engineering and ballistic measurements.
-
1 mph ≈ 1.467 ft/s
-
Special Units
-
Knots (nautical miles per hour): Used in aviation and maritime contexts.
-
1 knot ≈ 1.852 km/h or 1.15078 mph
-
-
Mach: Represents the speed of sound (Mach 1 = the speed of sound at sea level, about 343 m/s); used in aerospace and high-speed aviation.
Applications
-
Transportation: Vehicle speed limits, flight velocity, and train travel all rely on accurate speed measurement.
-
Physics and Engineering: Motion equations and simulations use SI units like m/s.
-
Weather and Meteorology: Wind speed is measured in km/h, m/s, or knots.
-
Sports: Athlete performance, such as sprint speed or pitch velocity, is tracked using standardized units.
Conversion Examples
-
1 m/s = 3.6 km/h
-
60 mph ≈ 96.56 km/h
-
100 km/h ≈ 62.14 mph
-
1 knot = 1.852 km/h = 1.15078 mph
Digital instruments such as GPS devices and speedometers automatically convert and display speed in preferred units based on the user’s region or application.
Importance of Standardized Units
Using standardized units of measurement for speed and velocity ensures clarity and consistency in navigation, engineering, and safety systems. From setting speed limits to launching spacecraft, accuracy in these measurements is critical.
Units of Measurement for Pressure and Force
Pressure and force are essential concepts in physics and engineering. Force measures the push or pull on an object, while pressure measures how that force is distributed over an area. Accurate units of measurement for both pressure and force are vital in fields like mechanics, aviation, automotive design, meteorology, and fluid dynamics.

Units of Measurement for Force
Force is a vector quantity defined by Newton’s second law:
Force = Mass × Acceleration
Metric Unit
-
Newton (N): The SI unit of force.
-
1 N = 1 kg·m/s²
It is the force required to accelerate 1 kilogram of mass by 1 meter per second squared.
-
Other Units
-
Kilonewton (kN): Commonly used in structural engineering and construction.
-
1 kN = 1,000 N
-
-
Dyne: Used in the CGS (centimeter–gram–second) system.
-
1 N = 100,000 dynes
-
-
Pound-force (lbf): Used in the U.S. customary system.
-
1 lbf ≈ 4.448 N
-
Units of Measurement for Pressure
Pressure is defined as force applied per unit area:
Pressure = Force ÷ Area
SI Unit
-
Pascal (Pa): The SI unit for pressure.
-
1 Pa = 1 N/m²
Very small in magnitude, so kilopascals (kPa) and megapascals (MPa) are more commonly used: -
1 kPa = 1,000 Pa
-
1 MPa = 1,000,000 Pa
-
Other Common Units
-
Bar: Widely used in meteorology and engineering.
-
1 bar = 100,000 Pa = 0.1 MPa
-
-
Atmosphere (atm): Refers to standard atmospheric pressure at sea level.
-
1 atm = 101,325 Pa
-
-
Pounds per square inch (psi): Common in the U.S. for tire pressure, hydraulics, and gas systems.
-
1 psi ≈ 6,894.76 Pa
-
Applications
-
Engineering: Pressure and force calculations ensure safe designs in structures, machines, and vehicles.
-
Meteorology: Atmospheric pressure is used to forecast weather and understand climate patterns.
-
Aviation and Aerospace: Cabin pressurization, fuel systems, and structural forces are carefully monitored using standardized units.
-
Everyday Life: Pumping car tires (psi), using hydraulic jacks (kPa/MPa), and even spray cans rely on pressure measurements.
Conversion Examples
-
1 atm = 101.325 kPa ≈ 14.7 psi
-
1 bar = 100 kPa
-
1 lbf = 4.448 N
-
1 psi = 6.895 kPa
Importance of Accuracy and Safety
Because incorrect pressure or force can lead to equipment failure or danger, using the correct units of measurement and precise instruments is vital. Engineers and technicians rely on these measurements to ensure performance and safety.
Units of Measurement for Energy and Power
Energy is the capacity to do work, while power is the rate at which energy is transferred or used. Both are foundational concepts in physics, engineering, and everyday life—especially when dealing with electricity, fuel, mechanical systems, or food. Understanding the correct units of measurement for energy and power is essential for everything from calculating electricity bills to designing engines and evaluating human calorie intake.
Units of Measurement for Energy
SI Unit
-
Joule (J): The standard SI unit of energy.
-
1 joule = the energy used when a force of one newton moves an object one meter (1 J = 1 N·m)
-
Larger Units
-
Kilojoule (kJ):
-
1 kJ = 1,000 joules
Commonly used in food energy and mechanical systems.
-
-
Megajoule (MJ):
-
1 MJ = 1,000,000 joules
Used in large-scale energy calculations, such as industrial or transportation energy needs.
-
Other Common Units
-
Calorie (cal): Often used in nutrition.
-
1 cal = 4.184 J
Food labels typically use kilocalories (kcal), where 1 kcal = 1,000 calories.
-
-
British Thermal Unit (BTU):
Used in heating and cooling systems, especially in the U.S.-
1 BTU ≈ 1,055 joules
-
-
Kilowatt-hour (kWh):
A practical unit for electricity consumption.-
1 kWh = 3.6 million joules (3.6 MJ)
Used on utility bills to measure how much electrical energy a home or business consumes.
-
Units of Measurement for Power
Power measures how quickly energy is used or transferred.
SI Unit
-
Watt (W):
-
1 watt = 1 joule per second (1 W = 1 J/s)
Describes how fast work is being done or energy is being consumed.
-
Larger Units
-
Kilowatt (kW):
-
1 kW = 1,000 W
Commonly used for engines, appliances, and electrical systems.
-
-
Megawatt (MW):
-
1 MW = 1,000,000 W
Used for power plants, large factories, or grid power.
-
Other Units
-
Horsepower (hp):
Mainly used for engines in cars and machinery.-
1 mechanical horsepower ≈ 745.7 watts
-
Applications of Energy and Power Units
-
Electricity: Power usage is calculated in kilowatts, and energy consumption is tracked in kilowatt-hours (kWh).
-
Nutrition: Caloric values in food help measure how much energy the body gains.
-
Industry and Engineering: Engineers use joules and watts to design safe and efficient machinery.
-
Automotive and Aerospace: Horsepower and kilowatts indicate engine performance and fuel efficiency.
-
HVAC and Appliances: BTUs and watts determine heating/cooling output and power usage.
Conversion Examples
-
1 kWh = 3.6 × 10⁶ J
-
1 kcal = 4,184 J
-
1 horsepower = 745.7 W
-
1 MJ = 277.78 Wh
Accurately using the correct units of measurement for energy and power is critical for managing resources, improving efficiency, and maintaining safety. From running a household appliance to fueling a rocket, these units are part of the core language of modern science and technology.
Units of Measurement for Electric Current and Voltage
Electricity powers modern life—from lighting and electronics to industrial machines and vehicles. Two fundamental quantities in electricity are electric current and voltage, each described using specific and standardized units of measurement. These units ensure safety, consistency, and compatibility in the design, operation, and maintenance of electrical systems.
Units of Measurement for Electric Current
Electric current refers to the flow of electric charge through a conductor, typically measured in terms of how much charge flows per second.
SI Unit: Ampere (A)
-
The ampere, symbol A, is the base SI unit of electric current.
-
Defined as one coulomb of electric charge passing through a point in a circuit per second.
-
Typical usage:
-
Small household devices: milliamperes (mA)
-
Industrial machines: amperes (A) or kiloamperes (kA)
-
Smaller Units
-
Milliampere (mA):
-
1 A = 1,000 mA
Used for measuring current in small electronic components and circuits.
-
Units of Measurement for Voltage
Voltage (also called electric potential difference) measures the energy required to move a unit of electric charge between two points.
SI Unit: Volt (V)
-
The volt, symbol V, is the standard unit of voltage.
-
Defined as the potential difference that will move one ampere of current using one watt of power.
-
Common voltage levels:
-
Household battery: 1.5 V
-
Wall outlets (US): 120 V; (Europe): 220–240 V
-
Industrial equipment: 480 V and above
-
Other Electrical Units (Related Concepts)
-
Ohm (Ω): Unit of electrical resistance
-
Defined by Ohm’s Law: V = I × R
-
-
Watt (W): Unit of electrical power
-
Power = Voltage × Current (P = V × I)
-
-
Coulomb (C): Unit of electric charge
-
1 ampere = 1 coulomb/second
-
Applications
-
Home and Building Wiring: Uses amperes and volts to size circuit breakers and select wiring.
-
Electronics: Low-voltage circuits use precise mA and V values for microchips and sensors.
-
Power Distribution: Engineers design substations and power grids using kilovolts (kV) and kiloamperes (kA).
-
Battery Technology: Battery ratings in volts and ampere-hours (Ah) define capacity and performance.
Conversion Examples
-
1,000 mA = 1 A
-
1 kV = 1,000 V
-
Ohm’s Law: V = I × R (Volts = Amps × Ohms)
-
Power Formula: P = V × I (Watts = Volts × Amps)
Importance of Standardized Units
Accurate use of units of measurement in electric current and voltage is essential to prevent circuit failures, fires, and electrocution. Standardization allows manufacturers, electricians, and engineers to safely design, operate, and troubleshoot electrical systems across the globe.
Standardization of Units of Measurement
Standardization of units of measurement is crucial for global consistency, precision, and safety in science, engineering, trade, and everyday life. Without universally accepted units, communication and collaboration across countries, industries, and disciplines would be chaotic and error-prone.
Why Standardization Matters
-
Global Consistency: Allows people from different regions and backgrounds to understand, replicate, and trust measurements.
-
Accuracy and Precision: Enables scientists and engineers to measure physical quantities with extreme precision using reliable references.
-
Fair Trade and Commerce: Ensures products are measured, packaged, and sold with correct quantities, protecting both producers and consumers.
-
Safety and Regulation: Prevents errors in fields such as medicine, construction, and energy where incorrect measurements can cause harm or failure.
The International System of Units (SI)
The International System of Units (SI), established in 1960 and maintained by the International Bureau of Weights and Measures (BIPM), is the globally recognized standard for units of measurement.
SI Base Units:
| Quantity | Unit | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Temperature | kelvin | K |
| Amount of substance | mole | mol |
| Luminous intensity | candela | cd |
These base units are used to derive other units (e.g., newton, joule, pascal) in a coherent and standardized way.
Key Organizations Involved in Standardization
-
BIPM (Bureau International des Poids et Mesures): Oversees the maintenance and promotion of SI units.
-
ISO (International Organization for Standardization): Sets international standards across many sectors, often referencing SI units.
-
NIST (National Institute of Standards and Technology – USA): Develops and promotes measurement standards in the U.S.
-
IEC (International Electrotechnical Commission): Develops standards for electrical, electronic, and related technologies.
Challenges in Standardization
-
Regional Preferences: Some countries still use imperial or customary systems (e.g., U.S., Myanmar), leading to dual-system complexity.
-
Historical Inertia: Long-standing habits and existing infrastructure slow down transitions to SI units.
-
Conversion Errors: Mistakes during unit conversion have caused serious accidents (e.g., the loss of NASA’s Mars Climate Orbiter due to a metric-imperial mix-up).
Benefits of Adopting SI Units Globally
-
Simplified Education and Training
-
Improved Product Compatibility
-
Streamlined Research and Development
-
Fewer Errors in International Trade
Standardizing units of measurement through systems like SI enhances clarity, safety, and cooperation in every field. As industries and nations continue to globalize, the importance of using and understanding standardized units becomes even more critical for accuracy, innovation, and economic progress.
Common Mistakes and Misunderstandings in Units of Measurement
Despite the widespread use of standardized units of measurement, errors and confusion still frequently occur. Understanding common mistakes can help prevent miscommunication, costly errors, and safety hazards in various fields.
1. Mixing Different Measurement Systems
One of the most common problems is confusing or combining units from different systems, such as mixing metric units (meters, kilograms) with imperial units (feet, pounds). This can lead to errors in calculations and product specifications.
Example:
The Mars Climate Orbiter mission failed because one engineering team used pounds-force seconds while another used newton seconds, causing the spacecraft to enter the Martian atmosphere incorrectly.
2. Incorrect Unit Conversion
Errors often happen during manual conversions between units. Using wrong conversion factors or neglecting to convert units properly can result in significant inaccuracies.
Example:
Confusing inches with centimeters or failing to convert time units (seconds to minutes) correctly in physics problems.
3. Misunderstanding Units with Similar Names
Some units sound alike but measure different quantities, leading to confusion.
-
Mass vs. Weight: Mass (kilograms) is often mistakenly called weight.
-
Calorie vs. Kilocalorie: Food energy is usually measured in kilocalories, but sometimes just “calorie” is used, causing misunderstanding.
4. Ignoring Unit Scale Differences
Units like millimeters, centimeters, meters, and kilometers differ by powers of ten, but overlooking these differences can cause large errors.
Example:
A 5 cm measurement mistakenly interpreted as 5 m leads to a tenfold error in length.
5. Assuming Units Without Specification
Sometimes measurements are given as numbers without clear units, which makes interpretation impossible or ambiguous.
6. Using Non-Standard or Obsolete Units
Using outdated or non-standard units can cause confusion, especially in scientific or international contexts.
7. Relying on Approximate Units for Precise Work
Using rough estimates instead of precise units (e.g., “a foot” without specifying exact inches or decimals) may be acceptable in casual contexts but leads to problems in engineering or manufacturing.
How to Avoid These Mistakes
-
Always specify units explicitly.
-
Double-check unit conversions using reliable references or digital tools.
-
Use standardized units (preferably SI) whenever possible.
-
Educate and train personnel on the importance of units and conversions.
-
Use software or calculators that automatically handle unit conversions.
Digital and Technological Advancements in Units of Measurement
The evolution of technology has significantly transformed how we measure, convert, and use units of measurement in everyday life and professional fields. Digital tools and modern technology have made measurement more precise, accessible, and convenient than ever before.
Digital Measurement Tools
-
Digital Scales and Sensors: Devices like electronic scales, laser distance meters, and digital thermometers provide highly accurate readings with immediate results, reducing human error.
-
Smart Instruments: IoT-enabled sensors continuously monitor physical quantities such as temperature, pressure, or flow in industrial processes, transmitting real-time data.
-
Wearables and Mobile Devices: Smartphones and smartwatches come equipped with sensors that measure steps, heart rate, speed, and altitude using standardized units, empowering users with health and activity data.
Software and Apps for Unit Conversion
-
Conversion Calculators: Many apps and online tools instantly convert between different units of measurement, supporting dozens of unit types—from length and volume to energy and data storage.
-
Engineering and Scientific Software: Programs like MATLAB, AutoCAD, and simulation software handle complex unit conversions automatically, allowing professionals to focus on analysis rather than calculations.
-
Integration with Data Systems: Measurement data from instruments can be integrated into larger data management or control systems, ensuring consistent unit use across platforms.
Advances in Measurement Standards
-
Atomic Clocks: Modern atomic clocks use vibrations of atoms like cesium or rubidium to define the second with unparalleled precision, supporting GPS and global timekeeping.
-
Quantum Metrology: Emerging technologies are redefining fundamental units based on physical constants, enhancing the stability and universality of measurements.
Impact on Industries
-
Manufacturing: Precision measurement ensures parts meet strict tolerances, reducing waste and improving quality.
-
Healthcare: Accurate dosage measurement and patient monitoring improve treatment outcomes.
-
Environmental Monitoring: Sensors track pollution, weather, and climate data, using standardized units to facilitate global collaboration.
-
Energy Management: Smart grids and meters measure energy consumption accurately, promoting efficiency and sustainability.
Benefits of Technological Advancements
-
Reduced human errors and inconsistencies
-
Faster and more reliable data collection
-
Enhanced ability to work with complex or multiple unit systems
-
Greater accessibility of measurement tools to the general public
Conclusion
Digital and technological innovations have revolutionized the way we interact with units of measurement, making them more precise, versatile, and integrated into modern life. As technology advances, measurement standards will continue to evolve, fostering greater accuracy and global interoperability.
Conclusion
Units of measurement are fundamental to understanding, describing, and interacting with the physical world. From the earliest body-based units to the highly precise standards of today, the evolution and standardization of units of measurement have enabled advances in science, technology, commerce, and daily life. Whether measuring length, mass, time, temperature, or electrical quantities, standardized units ensure clear communication and consistency across different fields and regions.
The widespread adoption of the International System of Units (SI) has unified measurements worldwide, reducing confusion and facilitating global collaboration. Meanwhile, technological advancements—from digital sensors to atomic clocks—have enhanced measurement accuracy, efficiency, and accessibility.
However, awareness of common mistakes and careful use of units remain critical to avoid costly errors. Understanding units, conversions, and their applications empowers individuals and professionals to make informed decisions and innovate confidently.
As we move forward, the role of units of measurement will only grow more important, supporting new technologies and deeper scientific discoveries. Mastery of this universal language of measurement is essential for progress in an interconnected and rapidly evolving world.